Drugmakers that dominate the world's insulin market must scale up access efforts globally
The three companies that dominate the global insulin market – Eli Lilly, Novo Nordisk and Sanofi – are pursuing a patchwork of strategies to expand access to their products in low- and middle-income countries (LMICs).
The report also examines initiatives from biosimilar manufacturer Biocon, shedding light on the potential role of biosimilar insulins in improving affordability and widening access.
Increasing access to analogue insulins, not just human insulins, should be a priority – so that people in LMICs have the same product choices that have been widely available to those in high-income countries for decades.
One hundred years after the ground-breaking discovery of insulin, persistent gaps in access to this highly effective drug remain critical. The number of people with diabetes worldwide is expected to reach 643 million by 2030, and 783 million by 2045 – rising most rapidly in low- and middle-income countries (LMICs), where there is stark inequity in access to insulin.
A new report by the Access to Medicine Foundation examines what Eli Lilly, Novo Nordisk and Sanofi are doing to expand access to their insulin products in LMICs, including both human and analogue insulins. Along with these three main insulin producers, the independent analysis* looks at Biocon, which is one of the largest producers of biosimilar insulins globally.
As the report shows, people with diabetes in LMICs are not currently getting the choice of insulins they need, and access to this life-saving drug is severely limited or lacking in many countries.
“While diabetes care has advanced since the discovery of insulin 100 years ago, this highly effective drug, and the range of products that help manage chronic diabetes, is only available to a fortunate few. Millions of people living in low- and middle-income countries still don’t have access to life-saving insulin, much less the choice of treatment available in high-income countries. This report highlights the urgent actions companies, governments and their partners can take to provide patients in resource-limited settings with the choice of treatment they deserve – no matter where they live.” – Jayasree K. Iyer, CEO, Access to Medicine Foundation.
Companies’ efforts are currently fragmented, and are often focused on a few countries, patient populations and products – falling short in addressing the extent of the global insulin inequity problem. However, the report identifies ways in which these companies are now seeking sustainable approaches to scaling up access in the long term.
Providing choice of treatment to all patients living with diabetes
It is vital that people with diabetes have access to a reliable and affordable supply of insulin in order to stay alive and healthy. Yet, in many countries, people still do not have access to human insulin – a form of insulin that was discovered almost half a century ago.
In addition to human insulin, people living with diabetes in LMICs should have access to the analogue insulin products that have become well-established and preferred by patients and healthcare professionals in higher-income countries over recent decades. Analogue insulins can allow for improved control of blood sugar levels after meals and overnight – making patients' lives easier, reducing the risk of hypoglycaemia (low blood glucose levels), and potentially increasing adherence to treatment. However, these products can cost over two to six times more than human insulins, with prices varying significantly between countries.
The World Health Organization (WHO) has already recognised the importance of analogue insulins, and not just human insulins, by adding several analogues and their biosimilars to the WHO Model List of Essential Medicines (EML) in 2021; a firm indication that they should be available to patients in every country.
One of the most alarming findings of the report is how limited access to insulins – human or analogue – currently is:
Only 29 of the 108 countries in scope have all the insulins from WHO's EML registered, and only one of those is a low-income country.
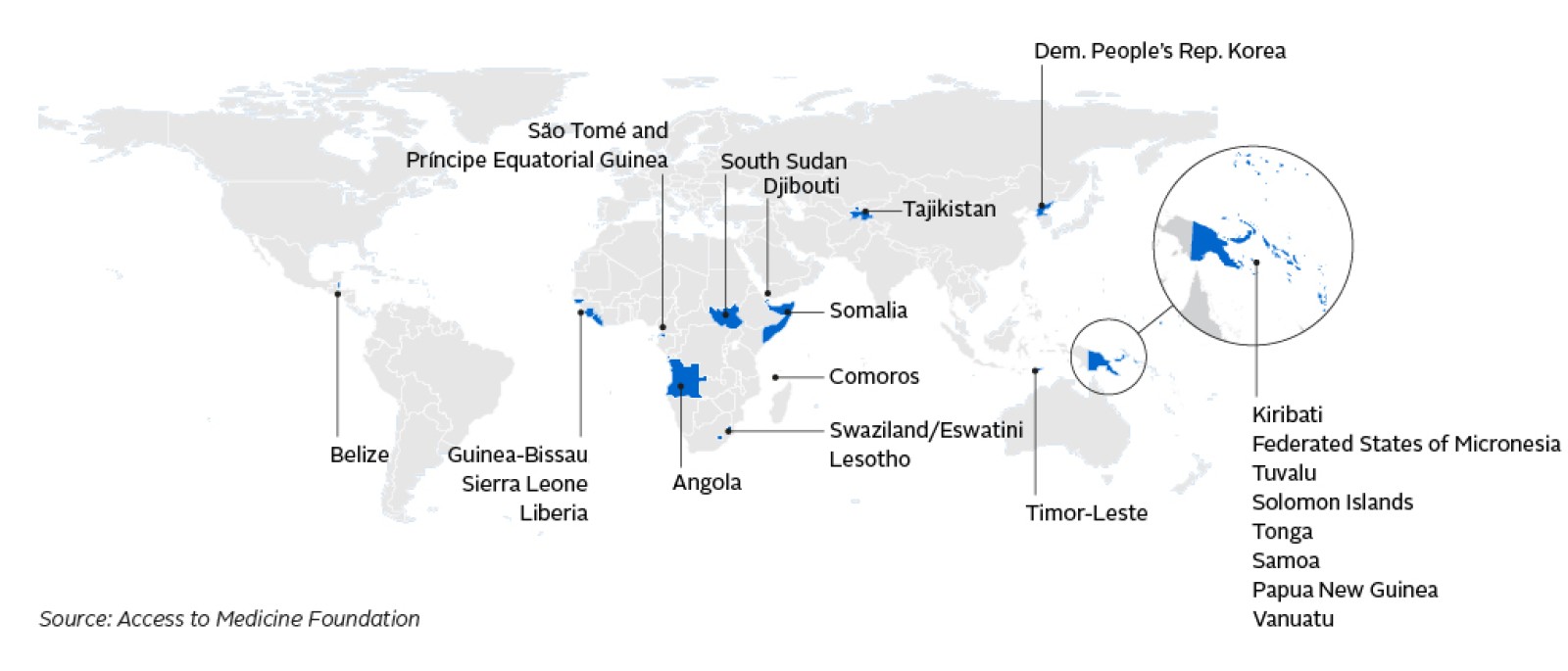
In 24 countries, no insulins were found to be registered at all.
Registering a medicine is a necessary step that enables its sale in a country. This means that people living in these countries have no access to insulin – and the stark reality is that without access to insulin, many more diabetic children and adults will suffer and die from this life-long, chronic disease.
Mapping out the 24 countries where no insulins were found to be registered
What are companies currently doing?
The report finds that the main insulin manufacturers are pursuing a patchwork of strategies to expand access to their products in LMICs, including paediatric programmes, price ceilings, and equitable pricing policies.
Eli Lilly, Novo Nordisk and Sanofi have diverse strategies in place to expand access to their products.
Notably, Novo Nordisk and Sanofi have recently taken steps to apply integrated business approaches encompassing some of their insulin products.
However, there are persistent gaps in access to all insulins; most affordability strategies remain primarily focused on human insulins and few are found for analogue products.
The full report, available for download now, contains a Company Profile for each of the insulin manufacturers in scope: Eli Lilly, Novo Nordisk, Sanofi and Biocon. This sets out what, specifically, each company is currently doing to expand access to its insulin products in LMICs.
Scaling up and diversifying the global insulin market
It is within these companies’ abilities to expand access if they build on the strategies they have already employed, with the report identifying ways in which they are now seeking sustainable approaches to scaling up access. Such approaches will need to be holistic and consider insulin within the broader contest of diabetes care, including patients’ access to all the products needed, including monitoring devices (glucometers and test strips) and delivery devices (syringes and injection pens).
Expanding the number and type of insulins on the market in LMICs will also have a positive impact on both availability and affordability. Although Eli Lilly, Novo Nordisk and Sanofi are dominant in the global insulin market – combined these companies control over 90% of the global market by value – several biosimilar companies are already producing a significant quantity of insulin products in LMICs.
The unrealised potential of biosimilars in expanding access to insulin
A biosimilar drug is a drug that is highly similar to a biological drug (such as insulin) that is already on the market. Quality-assured biosimilar insulins hold a great deal of as-yet unrealised potential, especially with several patents on long-acting insulins recently expiring – meaning biosimilar versions can now be launched as competitors.
On this front, Biocon is currently one of the largest global manufacturers of biosimilar insulin and has a wide footprint in LMICs. The company has ambitious goals to improve access to its insulins in these countries by lowering prices and boosting affordability, although it is in the early stages of taking steps towards achieving these goals.
Harnessing momentum across the global insulin ecosystem
Insulin manufacturers remain a critical link in the global chain of stakeholders needed to deliver access to diabetes care. Companies can take action by ensuring treatment options that are available in high-income countries are also available in LMICs, increased registration in LMICs of both human and analogue insulins, and at a minimum, ensuring that all essential insulins are registered in the poorest countries.
“Less than 30% of low- and middle-income countries currently have access to all insulins deemed essential, with the cost of insulin being a huge barrier to access. Insulin products, particularly analogue insulins, need to become affordable to every payer. Tapping into the promising potential of biosimilar insulins, for example, could expand affordability and access. While access to insulin is a complex, multifaceted issue, more efforts are needed on a greater scale, covering a wider range of products.” – Claudia Martínez, Research Programme Manager, Access to Medicine Foundation
To ensure affordable insulins are available to people in LMICs, companies can also pursue long-term financial sustainability. To achieve this, further collaborations are needed with governments and partners to integrate more access programmes into local health systems, alongside company affordability strategies and initiatives, particularly for analogue insulins.
While the report concludes that current access efforts fall short, there are signs that companies are now seeking sustainable ways to scale up access. However, systematic and sustainable approaches are required across the global insulin ecosystem in order to address the gaps in access to diabetes care worldwide.
More in the report
How Eli Lilly, Novo Nordisk, Sanofi and Biocon are addressing access to insulin
Lack of registration to essential insulins in LMICs and a patchwork of strategies to improve affordability
Access to insulin for children and young people
How to meet the challenge of expanding access to insulin
About this research
The Access to Medicine Foundation is an independent non-profit organisation that seeks to transform the healthcare ecosystem by motivating and mobilising companies to expand access to their essential healthcare products in low- and middle-income countries (LMICs). Products to treat diabetes, including insulins, have been in scope of the Foundation’s Access to Medicine Index since 2008. As of 2022, the Foundation has deepened its focus on diabetes care in LMICs This new study is part of a programme of activities that the Foundation has undertaken aimed at identifying new solutions for insulin companies to expand access to diabetes care, informed by a fresh assessment of current practice. The programme draws on the Foundation's long experience of engaging healthcare companies with global health priorities to drive the uptake of good practice by companies with a pivotal presence in the insulin market.
*The content in this paper has been drawn from data available in the public domain, peer-reviewed literature, and global health and policy reports. The paper was further informed by data collected for the 2022 Access to Medicine Index, as well as additional data collected specifically for the purpose of this publication and interviews held with partners and experts in the diabetes field. The content was supplemented with information provided by industry and global health stakeholders during the Expert Session ‘Closing the gaps in access to diabetes care in low- and middle-income countries’ hosted by the Foundation on 7 July 2022.



