New study from the Foundation analyses 10 years of data on pharma companies and access to medicine
The 10-year analysis finds clear evidence of progress, most notably in R&D, and in how companies approach access. A few companies are carrying the greater part of the load. Overall, companies’ activity concentrates on a few diseases and countries.
“Compared with ten years ago, pharmaceutical companies are taking seriously the problems people face in low- and middle-income countries when accessing healthcare. The situation is still fragile – a retreat by one company, or a drop in healthcare investments, will jeopardise the progress made so far.” Jayasree K. Iyer, Executive Director of the Access to Medicine Foundation.
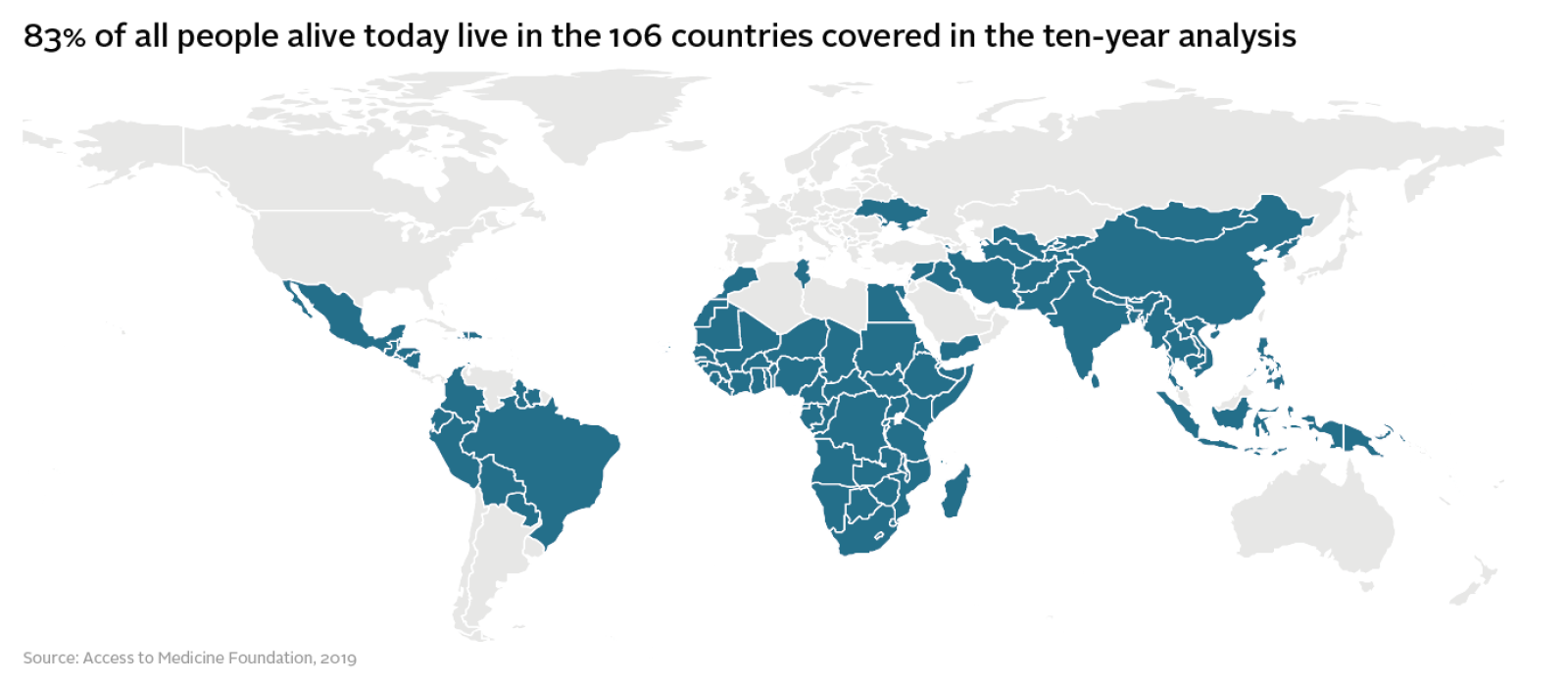
The report covers 20 of the world’s largest research-based pharmaceutical companies, which together account for 70% of global pharmaceutical revenues. They include highly diversified companies, such as GSK, Pfizer, Novartis and Sanofi, which all have large generics divisions. Several companies with a narrower focus, such as Gilead and Novo Nordisk, are also included in the cohort.
These companies have been tracked in the Access to Medicine Index since 2008. The Index is now the longest-running independent study of pharmaceutical company behaviour on access to medicine. Today’s report looks at the 47 diseases and conditions that have been consistently covered by the Index since 2014.
The report is the first independent study of its kind. It provides a springboard for discussions on how improvements can be sustained and expanded by the pharmaceutical industry in order to achieve SDG 3 on health and wellbeing by 2030.
The scale of the access challenge
Hundreds of millions of people live on very low incomes, and have no access to robust health systems, particularly people in Least Developed Countries and low-income groups in middle-income countries. This can be due to many factors, such as the affordability of medicines, lack of trained healthcare workers, or inefficient supply chains.
Action is being taken to turn this situation around. For example, child mortality dropped by almost 50% between 1990 and 2013. More than half of all people living with HIV/AIDS are accessing antiretroviral therapy.
Many stakeholders are playing a role in these milestones, not least governments and policy makers, as well as the non-profit and private sectors, including generic medicine manufacturers. Large research-based pharmaceutical companies have the ability to develop and bring new medicines to market at scale.
Pharma companies are gradually changing how they do business
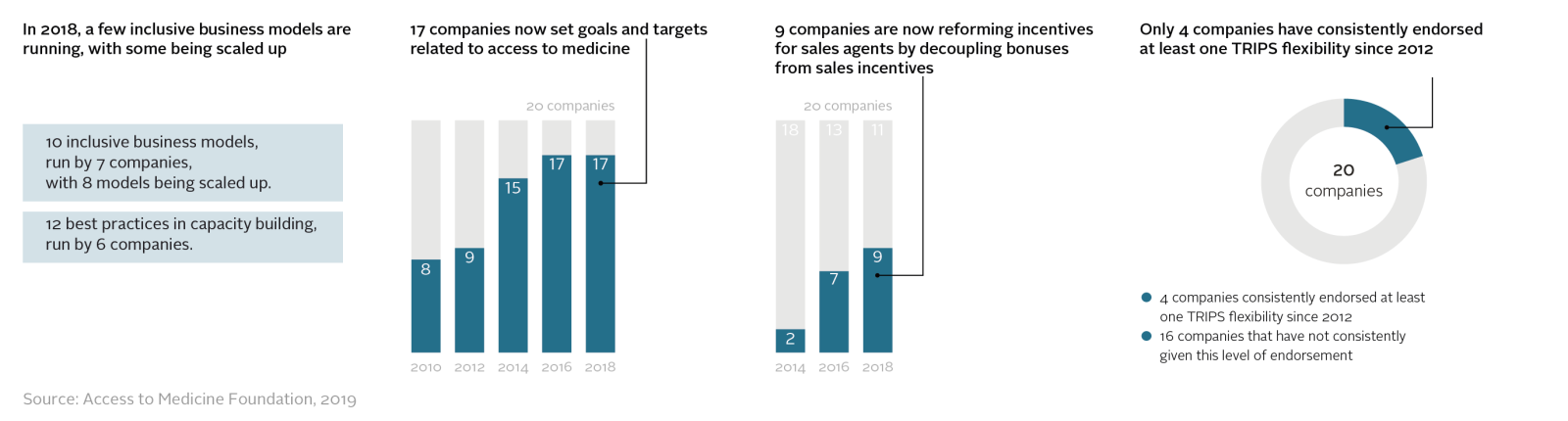
The report finds that several pharmaceutical companies are now doing business in new, inclusive ways that aim to reach people on very low incomes. Seven companies are running a total of ten inclusive business models, eight of which are being expanded. Plus, 17 companies now set measurable targets related to access to medicine, up from eight in 2010. Yet, only some companies (9) are tackling the risks of unethical sales behaviour by changing sales bonuses. Fewer companies (4) have consistently supported international trade agreements designed to ensure the poorest people can benefit from medical innovation.
R&D pipelines grow, particularly for key diseases such as malaria, HIV/AIDS and tuberculosis
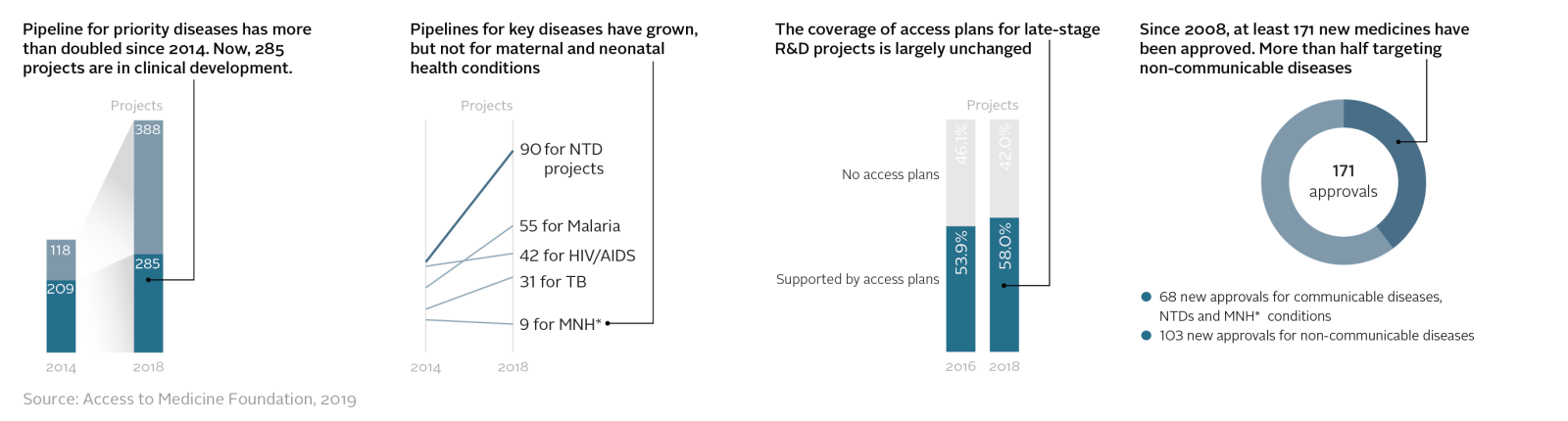
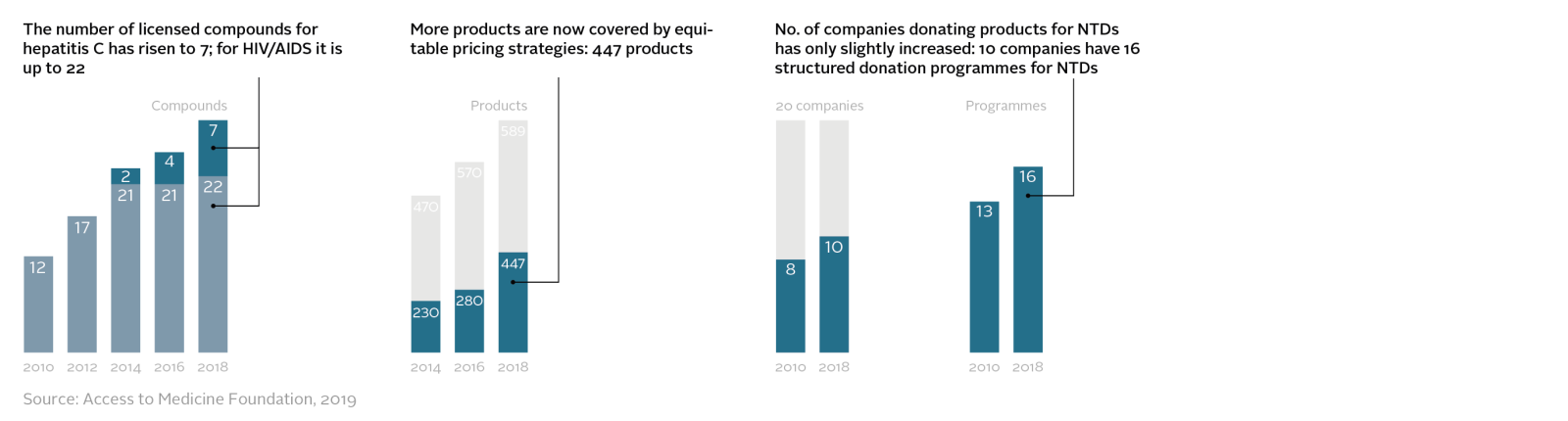
R&D pipelines have grown markedly, due to an effective recipe for engaging pharmaceutical companies in specific R&D challenges. The R&D pipeline has more than doubled since 2014 for a set of 47 high-burden and priority diseases, including HIV/AIDS, malaria and tuberculosis. Plus, five companies now systematically plan, as part of the R&D process, to address access to successful projects. In general, R&D activity focuses on commercial opportunities; more medicines for profitable non-communicable diseases were developed than medicines for diseases of poverty between 2008 and 2018 (103 new medicines for the 47 diseases in scope compared to 68).
Use of access tactics increases, but many products are not yet covered
Pharmaceutical companies have three main tactics for improving access to health products: pricing, licensing and donations. All three tactics are being used more frequently than before and in pro-access ways. For example, access-oriented licensing has expanded steadily since 2010, now covering 29 compounds, including a complete suite of HIV/AIDS and hepatitis C treatments. In the future, the use of these tactics has potential to expand to many more products in additional countries, so more people can benefit.
The factors driving change
The report shows that, in specific areas, more companies are taking action to improve global health than ten years ago. It also identifies an effective recipe for bringing pharmaceutical companies on board. This recipe consists of setting clear priorities endorsed by the international community of experts in global health. It also requires publicly funded mechanisms for reducing the risks companies face when investing in global health, or for shaping markets with little prospect of profitability. The recipe also calls for long-term and coordinated financial support from multiple donors, and a sustained investment in health from national governments, including to support healthy markets.
However, notes the report, this recipe is currently being used for only a few diseases and conditions, such as neglected tropical diseases (NTDs), HIV/AIDS and child and maternal mortality. Similarly, the uptake of good practice is generally confined to a comparatively small group of pioneering companies, a few countries, and on a few diseases, such as NTDs, HIV/AIDS, malaria and tuberculosis.
What next?
In the coming months, the Access to Medicine Foundation will use the findings as it identifies what the main focus on access to medicine should be for the pharmaceutical industry and its partners in the next decade, through discussions with leading experts working to improve access to medicine. The core question will be how to sustain a response to global health challenges at scale amidst increasing pressures.
“Going forward, the big challenge for all of us working on access is to match the scale of action to the scale of the problem.” Jayasree K. Iyer, Executive Director, Access to Medicine Foundation.
Read Jayasree K. Iyer's view on what this research means for the pharmaceutical industry and access to medicine in the next 10 years. Share your ideas on what must happen to achieve progress at scale amidst increasing pressures.
About the Access to Medicine Foundation
The Access to Medicine Foundation is an independent non-profit organisation based in the Netherlands, which analyses how the world's largest pharmaceutical companies are addressing access to medicine. Its mission is to stimulate and guide pharmaceutical companies to do more for the people who live in low- and middle-income countries. The Foundation defines the actions pharmaceutical companies can and should be taking through a process of stakeholder consensus building, gathering the views of independent experts in global health, access to medicine and the industry. The Foundation is funded by UK Aid, the Dutch Ministry of Foreign Affairs, the Bill & Melinda Gates Foundation and the Dutch Ministry of Health, Welfare and Sport.