Signs of progress by pharma against superbugs, yet little movement to address access
The 2021 Benchmark identifies bright spots of progress in how the pharmaceutical industry is responding to the threat of drug resistance.
However, there is a lack of momentum when it comes to making the right antibiotics accessible for people in low- and middle-income countries, where the risk of drug-resistant infections is highest.
The Benchmark examines 17 companies, producing more than 800 antibiotics at 1,057 manufacturing sites, and with 92 R&D projects in the pipeline.
While there are many ways for pharma companies to improve access to vital antibiotics and antifungals in poorer countries, these are not being widely used, finds the 2021 Antimicrobial Resistance Benchmark. Nevertheless, in other areas, good practice is becoming more common. This is most notable in the growth in planning, during R&D, to ensure wider access to and responsible use of future products, as well as in the steps that companies are taking to curb the release of antibiotic waste into the environment.
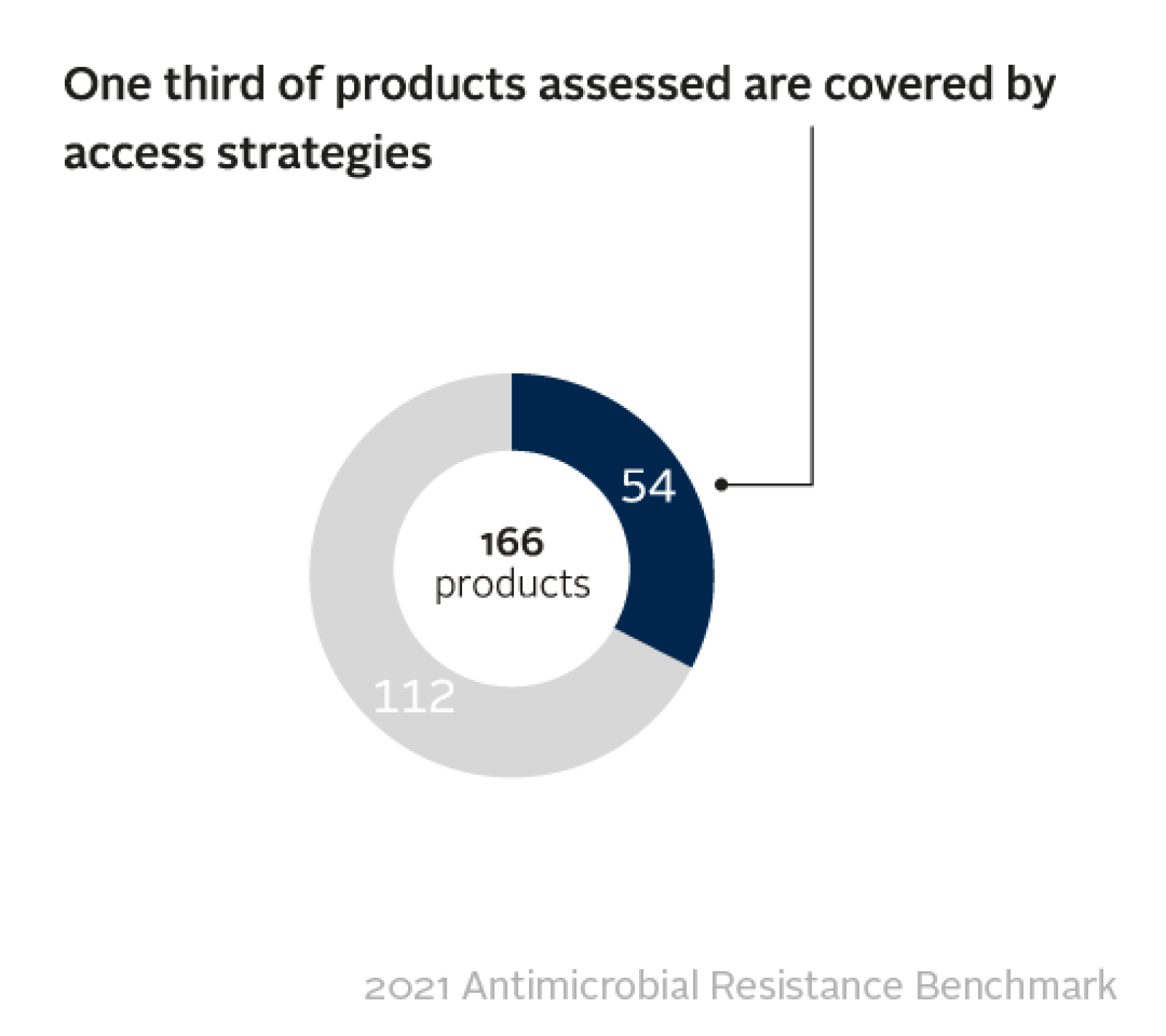
Out of 166 products examined, just one third have any kind of access strategy in place – such as price adjustments to make medicines more affordable, or licensing agreements to boost supply for low- and middle-income countries. This analysis includes more than 100 off-patent generic medicines identified by the World Health Organization as 'essential' to the good functioning of every health system.
However, there are examples that show the way forward. Several of the 17 companies evaluated – 8 large research-based companies and 9 generic medicine manufacturers – are taking tangible actions to improve access to specific products. This includes via technology transfers to manufacturing sites in countries including Pakistan, Brazil and Nigeria.
“The people who face the highest risk of infection and the highest rates of drug resistance have the hardest time getting the antibiotics they need. To redress this lack of equity, pharma must expand its focus beyond stewardship and the hunt for replacement antibiotics. Access to these new medicines, as well as those already on the market, must get the same level of attention”
Greater efforts to curb manufacturing impact
On the manufacturing side, pharma companies are making greater efforts to curb the release of antibacterials into local waterways, including by enforcing environmental standards with their third-party suppliers of active pharmaceutical ingredients (APIs). The 17 pharmaceutical companies evaluated by the Benchmark are manufacturing a combined total of 801 antibacterial products and report a total of 1,057 manufacturing sites in their supply chains, including those operated by third-party suppliers. Release of active pharmaceutical ingredients (APIs) into the local environment, for example into rivers, exposes local populations to unnecessary doses of antibacterials, and can potentially lead to newly resistant bacteria that pose a global threat.
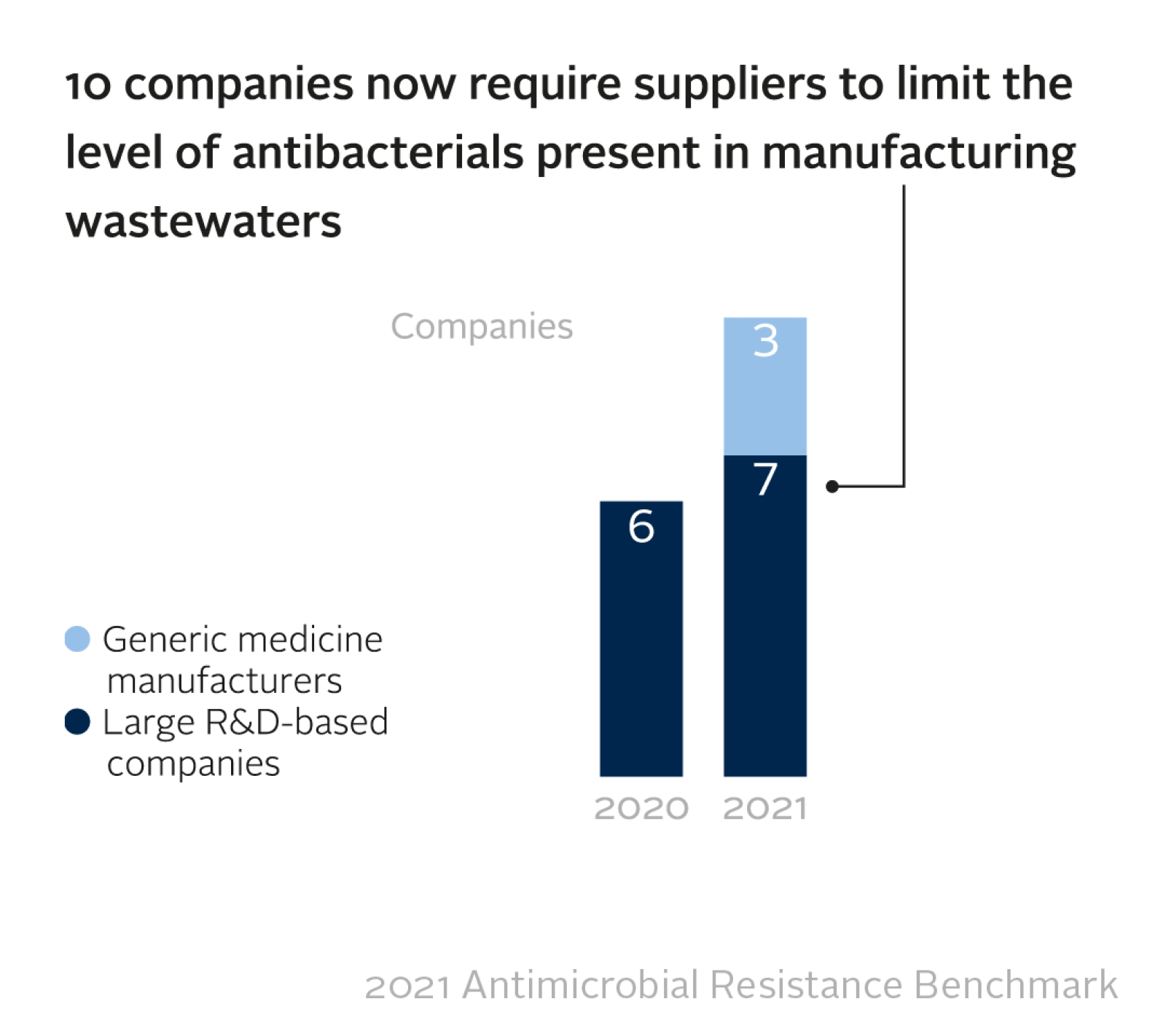
The companies that provided data report that limits apply at 561 of their suppliers’ manufacturing sites (64% of the combined total of 870). Although only 45 (5.2%) of sites were reported as actually being compliant, it is encouraging that companies are starting to exert positive influence on suppliers' standards.
Drug resistance is increasing
An estimated 750,000 people die each year due to drug-resistant infections. Yet by comparison, 5.7 million people die from treatable infectious diseases, due to a lack of access to medicines. Most of these people most live in low- and middle-income countries. Driven by natural selection, the emergence of drug resistance is inevitable. It can be slowed, provided antimicrobials are used responsibly and the spread of infection is controlled, for example through vaccination programmes. However, following decades of careless exposure to antibiotics, superbugs are now widespread and, warns the World Health Organization (WHO), represent a “global crisis that threatens a century of progress in health”.
R&D pipeline grows modestly
Big Pharma’s engagement with antibacterial and antifungal R&D appears to have stabilised after years of engagement by civil society and national governments to spur the development of replacement medicines. There are potential game-changers being tested by the companies evaluated, including vaccines for drug-resistant gonorrhoea, E. coli and C. difficile, among others.
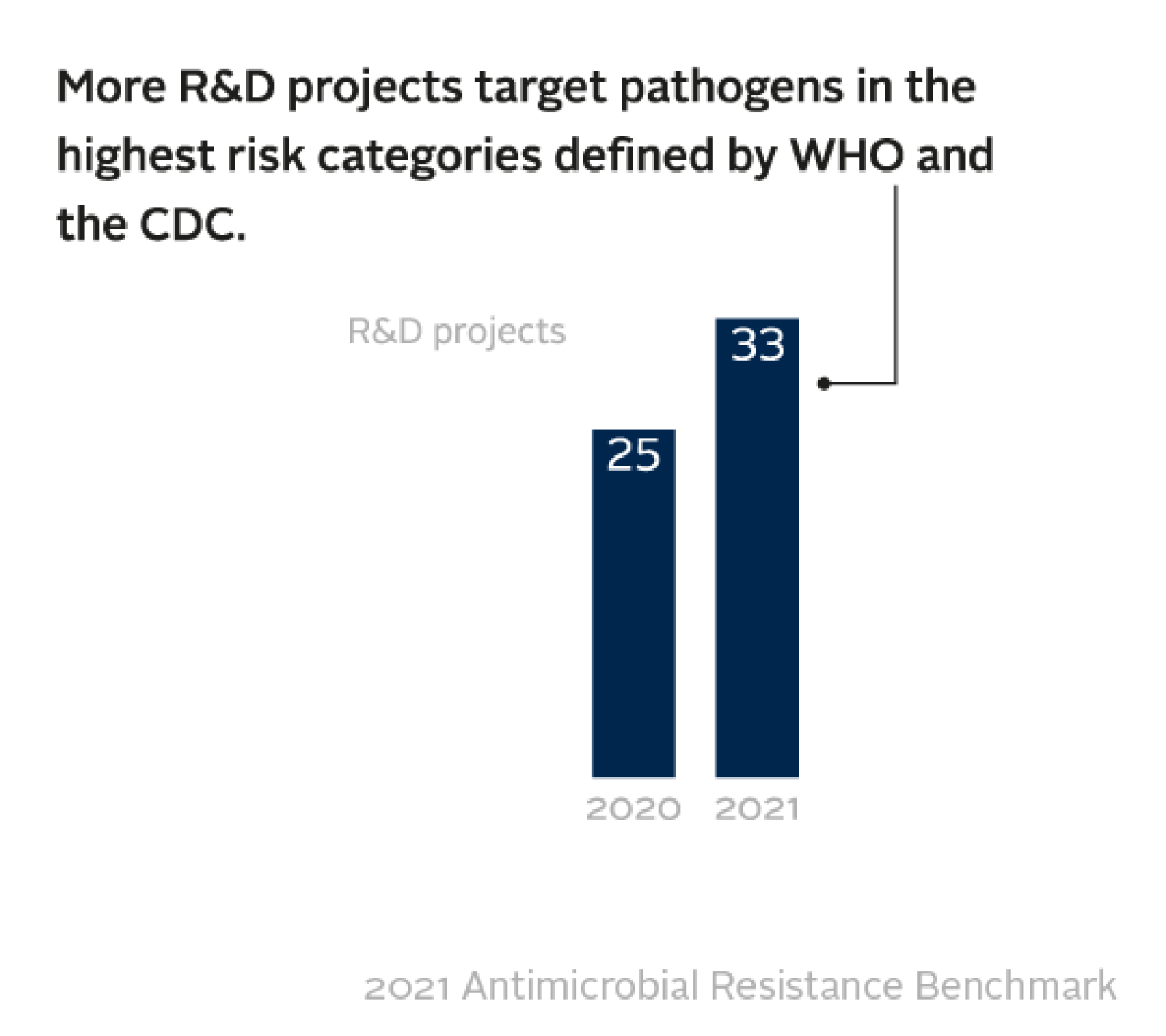
Among the eight large research-based pharma companies evaluated for the R&D activities, the Benchmark identified 92 projects that target infections caused by bacteria and fungi posing the highest risk of drug resistance. This is a slight increase on 2020, when the same eight companies were developing 77 projects. Nevertheless, the pipeline of replacement antibiotics remains small.
Encouragingly, new medicines and vaccines are more likely to quickly reach the people who need them. The Benchmark reports a significant uptick in companies planning ahead to make new products available in low- and middle-income countries quickly and responsibly. Such planning is becoming standard practice during late-stage development.
18 out of 20 late-stage medicine projects in this analysis have both access and stewardship plans in place, and all 11 late-stage vaccine projects have access plans. With limited products in late-stage development, it is essential that each new product is protected from misuse and overuse so that it stays effective for as long as possible.
Which companies lead efforts against resistance?
When comparing how companies perform, GSK and Pfizer are joint leaders among the large research-based companies evaluated. Pfizer has made the biggest strides since the previous Benchmark report, even during the COVID-19 pandemic, including by expanding its R&D pipeline of new antibiotics and antifungals.
Aurobindo, Abbott and Viatris are the leaders among the generic medicine manufacturers, taking steps to combat overselling of antimicrobials. Abbott and Viatris, alongside Cipla, are also the first of this group of companies to report setting discharge limits at their third-party suppliers’ manufacturing sites. Overall, progress by the generic medicine manufacturers can be seen in transparency, stewardship, sales practices, and wider registration of medicines in LMICs.
Stewardship practices, which focus on ensuring antimicrobials are used responsibly, have been expanded by almost all companies evaluated. Notably, in 2021, three more generic medicine manufacturers took action to combat the overselling of these medicines to healthcare professionals: Abbott, Aurobindo and Viatris. In 2020, the Benchmark also reported an increase in the number of pharma companies that had stopped the use of sales agents altogether, or which decoupled agents’ bonuses from sales volume.
“We have been tracking pharma’s response to drug resistance for five years. The latest Benchmark report shows that progress is possible, but change must accelerate.”
About the Benchmark
This is the third AMR Benchmark. It follows previous reports published in 2018 and 2020, and draws on information gathered since 2017. For this latest report, researchers at the Access to Medicine Foundation have collected and investigated data on 17 of the world’s largest pharmaceutical companies that produce antibiotics and antifungals (by volume and value of sales).
To assess access efforts, the Benchmark looks for all 17 companies’ actions to improve access in 102 low- and middle-income countries. To assess R&D, the Benchmark examines eight large research-based pharma companies’ projects targeting those pathogens that pose the highest risk of drug resistance, according to the US Centers for Disease Control (CDC) and WHO. It evaluates companies’ efforts to improve access to medicine by focusing only on those products where companies have a dominant market position, either due to patent protections or market share. The Benchmark is funded by the UK and Dutch governments, AXA Investment Managers and Wellcome Trust.