Harnessing Africa’s untapped clinical trial potential
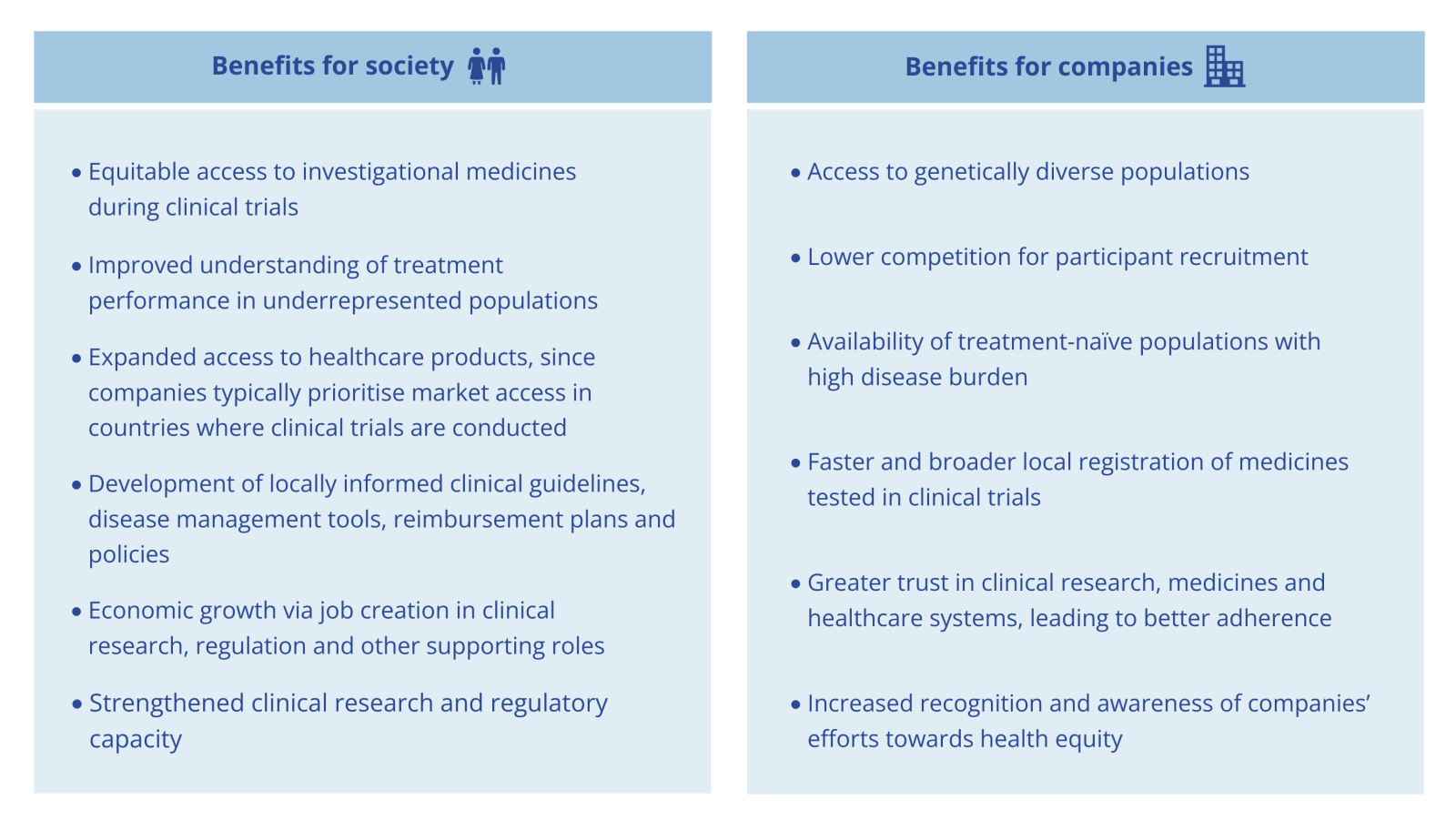
Clinical trials are a vital gateway to access to medicines, as companies often prioritise market access in countries where trials are conducted. Yet Africa, home to 19% of the world’s population and one-quarter of the global disease burden, hosted only 1.1% of all clinical trials in 2023. By expanding clinical research across the continent, the gap between disease burden and clinical trial representation can be bridged, delivering benefits for both people and industry.
A sparse and unequal map of clinical trials in Africa*
Industry-sponsored clinical trials across Africa are heavily concentrated in South Africa. Expanding trials to other countries with existing capacity, alongside South Africa, can help bridge chronic clinical research equity gaps and support more inclusive trial designs that reflect diverse populations. To help companies navigate this evolving landscape – particularly as geopolitical factors increasingly affect African clinical research centres that rely on foreign aid – three key enablers can guide more informed decision-making and reduce the risks associated with clinical trial expansion in Africa.
Enabler #1: Existing clinical trial sites
Limited local capacity – such as infrastructure and clinical expertise – has been cited as a barrier to expanding trials across Africa. Although it is currently not realistic to expect research to take place in every country, data from partners indicate that several countries, alongside South Africa, already have capacity that can be built on.
To reduce uncertainties and support expansion, companies can draw on publicly available resources such as the Clinical Trials Community (CTC) platform2 and BIO Ventures for Global Health (BVGH) platforms,3 including the African Consortium for Cancer Clinical Trials (AC3T)4 and the infectious disease-focused Global Innovation Alliance Network of Trial Sites (GIANTS).5 These tools offer varying levels of detail on trial sites and operational capacities. While not exhaustive, they can help inform decisions and make broader clinical trial activity across Africa more feasible (see Figure 3).
Some companies have already demonstrated their ability to utilise existing capacity across a range of African countries. For example, over the past ten years, Novartis has included participants from more African countries than any other company across its trials. They span multiple disease areas and 19 countries in total – 18 of which are documented as having clinical trial capacity by the BVGH and CTC platforms.
Expanding across active oncology sites
Clinical trials in Africa have primarily focused on neglected tropical diseases and a narrow set of infectious diseases, particularly malaria, tuberculosis and HIV. At the same time, clear opportunities exist to expand clinical research into areas such as novel antibiotics and non-communicable diseases – including sickle cell disease and cancer.
In discussions with the Foundation regarding the 2024 Index, companies expressed interest in identifying where capacity exists to conduct oncology trials. Over the past decade, around 29% of all Phase II-IV industry-sponsored clinical trials have focused on oncology treatments, highlighting the pharmaceutical sector’s strong interest in this disease area. Despite this, cancer remains largely overlooked on the continent, with only 3% of these oncology trials including participants from at least one African country during the same period.
Data from BVGH’s AC3T platform indicate that there are 62 sites across 21 sub-Saharan countries with the capacity to conduct oncology trials and established partners or experts on the ground (see Figure 3). However, over the last decade, companies have conducted oncology trials in only nine of these countries, underscoring significant untapped potential for company expansion. Merck & Co., Inc. (MSD) has led the most Phase II-IV oncology trials involving at least one African country, with Roche following closely behind (39 and 30 respectively). While MSD conducted all its oncology trials exclusively in South Africa, Roche has pursued a broader geographic reach across the continent, conducting trials in six sub-Saharan countries, all with documented clinical trial capacity identified by the BVGH’s AC3T platform.
Enabler #2: Growing regulatory capacity
Over the last few years, several African countries have strengthened their regulatory capacity by establishing well-functioning systems and regional hubs with specific clinical trial expertise, signalling their readiness to host trials (see Figure 4). This growing expertise lowers barriers to trial expansion, reduces operational risk and allows companies to broaden their clinical trial activities across the continent.
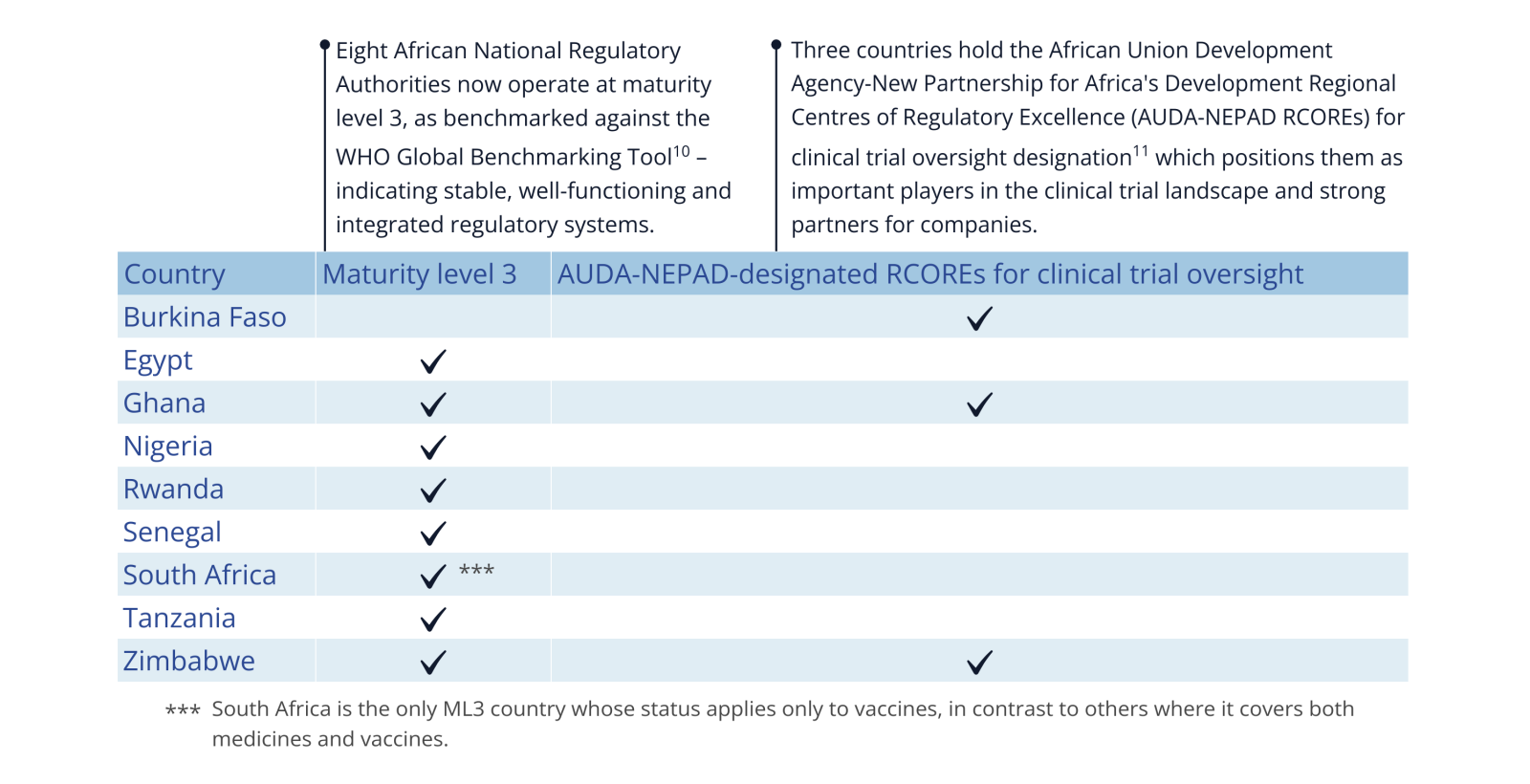
Ghana, for example, is currently designated as operating at maturity level 3 (ML3)10 and recognised as a Regional Centre of Regulatory Excellence (RCORE)11 for clinical trial oversight. Prior to receiving its ML3 status in 2020, five industry-sponsored clinical trials included participants from the country (2015-2019). Since then, participants in Ghana have been included in 17 industry-sponsored clinical trials – more than a threefold increase – covering a broader range of disease areas, with a notable rise in trials for sickle cell disease.
As Africa continues to grow its clinical trial capacity, companies can capitalise on harmonised processes. A key milestone is the establishment of the African Medicines Agency (AMA), now moving closer to full operation. In parallel, the African Medicines Regulatory Harmonization (AMRH) – an AUDA-NEPAD initiative and a precursor to AMA – has advanced the same goals. Within this framework, the African Vaccine Regulatory Forum (AVAREF) supports companies by streamlining national regulatory and ethics reviews through joint assessments of clinical trial applications for vaccines, medical products and devices across multiple African countries.12
Enabler #3: A broad network of partners
Working with partners and consortia can help overcome the barriers to expanding clinical trials in Africa. Several organisations are already active in building local capacity, including Science for Africa Foundation and its Clinical Trials Community Africa Network (CTCAN),13 as well as the Africa Clinical Research Network (ACRN),14 BIO Ventures for Global Health (BVGH)3 and the European & Developing Countries Clinical Trials Partnership (EDCTP),15 among others. These organisations have recently launched or expanded their programmes, forming a growing network that supports clinical trial expansion across the continent. For instance, in August 2025, ACRN issued an Expression of Interest, designed to ultimately connect more research sites, investigators and clinicians across the continent with global health and industry sponsors.
A strong example of how a company can collaborate with global and regional partners to conduct clinical trials in Africa is the paediatric arpraziquantel Phase III pivotal trial for schistosomiasis, spearheaded by the Pediatric Praziquantel Consortium.16 Sponsored by Merck KGaA, a founding partner of the Consortium, and supported by EDCTP and Global Health Innovative Technology Fund, the trial took place in Kenya and Côte d’Ivoire and was completed in 2021. Its results informed the European Medicines Agency’s positive scientific opinion in 2023 under the EU-M4all procedure,17 which led to the medicine’s inclusion on the WHO prequalification list in May 2024 and its first marketing authorisation in Tanzania in August 2025. The medicine was also recently included in the WHO Essential Medicines List (EML) and Essential Medicines List for Children (EMLc).
In parallel, in March 2025, Uganda became the first country to administer arpraziquantel to preschool-aged children through its national Neglected Tropical Disease Mass Drug Administration platform. The rollout has since expanded to Côte d’Ivoire and Kenya, with Senegal, Tanzania and other African countries expected to join subsequently.18
Such collaboration demonstrates how coordinated efforts between companies and partners can advance clinical research and improve access on the continent.
Clinical trials as pathways to better access in Africa
Broadening Africa’s clinical trial footprint and extending it beyond the diseases that have historically received the most attention on the continent is critical, especially given the growing impact of foreign aid cuts on clinical research in Africa. Expanding trials into underutilised regions with clinical trial capacity offers companies an opportunity to accelerate participant recruitment and shorten pathways to future market entry. At the same time, companies’ efforts can improve health outcomes through earlier access to investigational medicines, deeper understanding of how diverse populations respond to new therapies and enhanced post-approval access. By acting now, companies can seize a strategic opportunity that delivers both operational advantages and health impact in high-need regions.
The continent’s untapped potential is evident in its growing clinical trial capacity, maturing regulatory frameworks and developing network of partners. Together, the enablers outlined in this review provide a springboard for clinical research expansion that can help overcome the structural barriers the industry has faced in Africa and serve as practical steps to turn the continent’s potential into action.
*The analyses in this review were based on ClinicalTrials.gov (https://clinicaltrials.gov/) data. Phase II-IV industry-sponsored clinical trials published from 01 January 2015 to 22 July 2025 were included. Data extraction was completed between July 2025 and August 2025.
References
Katte JC, Squires S, Dehayem MY, et al. Non-autoimmune, insulin-deficient diabetes in children and young adults in Africa: evidence from the Young-Onset Diabetes in sub-Saharan Africa (YODA) cross-sectional study. Lancet Diabetes Endocrinol. Published online September 1, 2025. doi:10.1016/S2213-8587(25)00120-2
Clinical Trial Community Africa. CTC Platform. Accessed September 1, 2025. https://ctc.africa/
BIO Ventures for Global Health. Accessed August 27, 2025. https://bvgh.org/
African Consortium for Cancer Clinical Trials - BIO Ventures for Global Health. Accessed August 27, 2025. https://bvgh.org/african-consortium-for-cancer-clinical-trials/
Global Innovation Alliance Network of Trial Sites - BIO Ventures for Global Health. Accessed August 27, 2025. https://bvgh.org/global-innovation-alliance-network-of-trial-sites/
Novartis Pharmaceuticals. Efficacy, Safety and Tolerability of KLU156 in Adults and Children ≥ 5 kg Body Weight With Uncomplicated P. Falciparum Malaria (KALUMA). ClinicalTrials.gov identifier: NCT05842954. Accessed August 27, 2025. https://clinicaltrials.gov/study/NCT05842954?term=Novartis%20coartem&rank=2
Pfizer. A Study to Assess the Safety and Efficacy of Inclacumab in Participants With Sickle Cell Disease Experiencing Vaso-occlusive Crises. ClinicalTrials.gov identifier: NCT04935879. Accessed August 27, 2025. https://clinicaltrials.gov/study/NCT04935879?term=NCT04935879&rank=1
AstraZeneca. Effect of Ticagrelor vs. Placebo in the Reduction of Vaso-occlusive Crises in Pediatric Patients With Sickle Cell Disease (HESTIA3). ClinicalTrials.gov identifier: NCT03615924. Accessed August 27, 2025. https://clinicaltrials.gov/study/NCT03615924?term=NCT03615924&rank=1
Hoffmann-La Roche. A Study Evaluating Atezolizumab and Bevacizumab, With or Without Tiragolumab, in Participants With Untreated Locally Advanced or Metastatic Hepatocellular Carcinoma (IMbrave152) (SKYSCRAPER-14). CliniaclTrials.gov identifier: NCT05904886. Accessed August 27, 2025. https://clinicaltrials.gov/study/NCT05904886?term=NCT05904886&rank=1
World Health Organization (WHO). List of National Regulatory Authorities (NRAs) operating at maturity level 3 (ML3) and maturity level 4 (ML4). December 20, 2024. Accessed August 27, 2025. https://www.who.int/publications/m/item/list-of-nras-operating-at-ml3-and-ml4
Regional Centres of Regulatory Excellence (RCOREs) | AUDA-NEPAD. Accessed August 27, 2025. https://www.nepad.org/publication/regional-centres-of-regulatory-excellence-rcores
African Vaccine Regulatory Forum Technical Committee (AVAREF-TC) | AUDA-NEPAD-AMRH. Accessed August 27, 2025. https://amrh.nepad.org/african-vaccine-regulatory-forum-technical-committee-avaref-tc
Clinical Trial Community Africa Network (CTCAN). Accessed August 27, 2025. https://www.ctcan.africa/
Africa Clinical Research Network (ACRN). Accessed August 27, 2025. https://acrnhealth.com/
The European & Developing Countries Clinical Trials Partnership (EDCTP). Accessed August 27, 2025. https://www.edctp.org/
Merck KGaA. L-PZQ ODT in Schistosoma Infected Children. ClinicalTrials.gov identifier: NCT03845140. Accessed August 27, 2025. https://clinicaltrials.gov/study/NCT03845140
Merck KGaA. Positive CHMP Opinion by EMA for Arpraziquantel to Treat Schistosomiasis in Preschool-Aged Children.; 2023. www.merckgroup.com
Pediatric Praziquantel Consortium. PRESS RELEASE First Preschool-Aged Child Receives Arpraziquantel for the Treatment of Schistosomiasis. www.pediatricpraziquantelconsortium.org
