The Bulletin of WHO publishes an article on the thematic study: hepatitis C by the Access to Medicine Foundation
An estimated 185 million people worldwide are infected with hepatitis C. The vast majority live in low- and middle-income countries, and as many as 500,000 people die of this disease each year. Thanks to a new generation of hepatitis C drugs, however, we now have the first real possibility of controlling this epidemic. Treatment is now much more effective, faster and easier to take. In May 2015, the World Health Organization (WHO) added six of these drugs to the WHO Essential Medicines List, sending an important signal about their importance and the need for them to be made widely available.
There has been considerable debate about whether the companies that own these hepatitis drugs are doing enough to make them accessible and affordable for the people who need them. The Access to Medicine Foundation has addressed this knowledge gap in its most recent research, which was published by the Bulletin of the World Health Organization. It comprises the first review of the activities being undertaken by the world’s largest pharmaceutical companies to ensure the accessibility of their hepatitis C drugs.
“This analysis is an important step in assessing what else can be done to improve access to these life-saving drugs,” says Danny Edwards, lead author of the study and Research Programme Manager at the Access to Medicine Foundation. “While some companies are making steps in the right direction, others are yet to share evidence of access strategies for their innovative hepatitis C drugs. This is concerning, given the fact these drugs represent both a cure and a genuine chance to curb the epidemic. Companies have important roles to play in supporting affordability and securing supply.”
Findings: limited evidence of engagement in access issues
Six of the world’s largest pharmaceutical companies are marketing medicines for hepatitis C: AbbVie, Bristol-Myers Squibb, Gilead, Johnson & Johnson, Merck & Co. and Roche.
These same six companies are developing almost 30 potential new treatments in total, ten of which are in Phase 3.
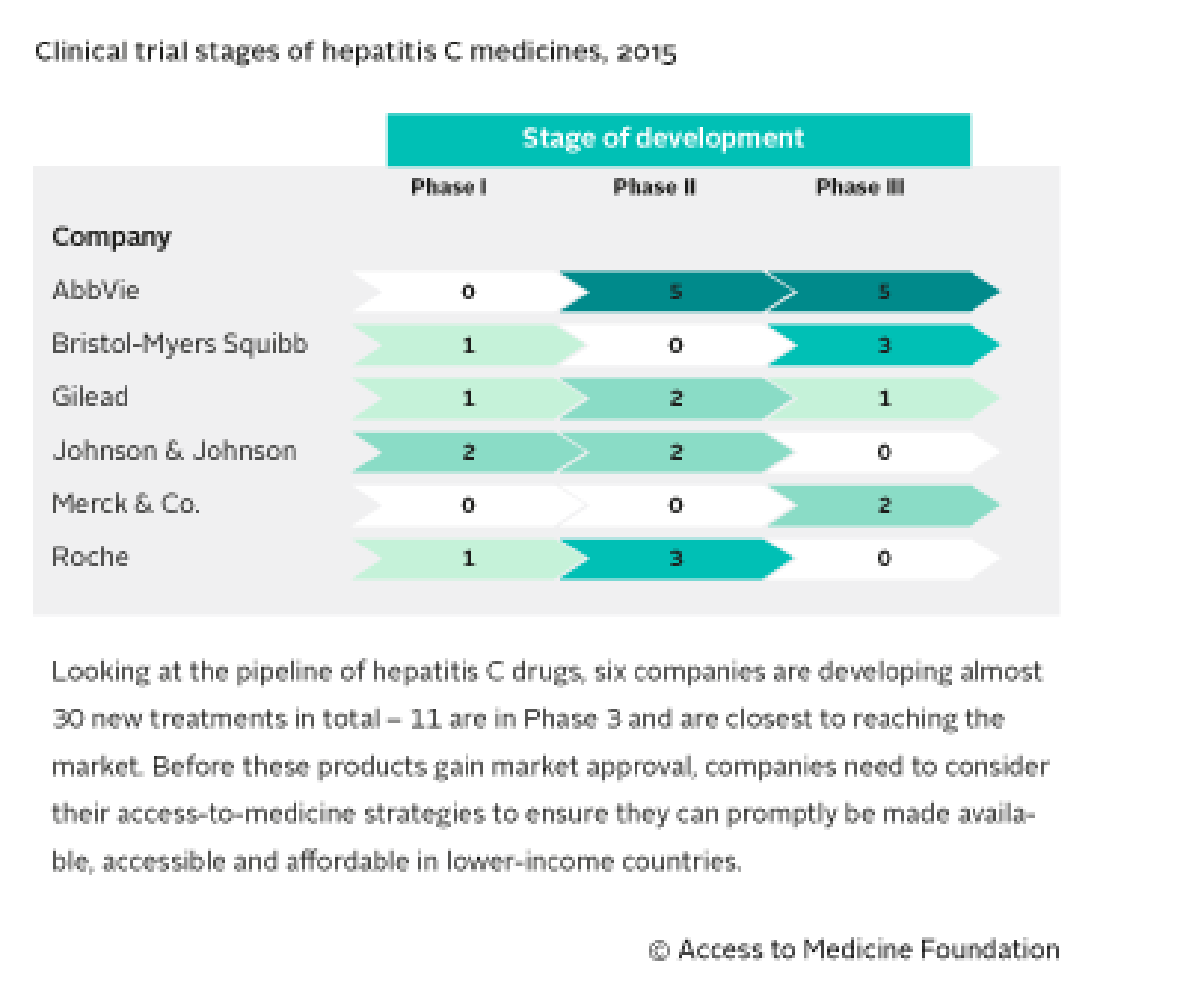
Five companies are marketing next-generation medicines: AbbVie, Bristol-Myers Squibb, Gilead, Johnson & Johnson and Merck & Co. Access strategies are in place for only two of these products: Sovaldi® (sofosbuvir) and Harvoni® (sofosbuvir/ledipasvir) (both Gilead). Merck & Co. and Roche are also marketing older, first-generation treatments.
Three companies have differential pricing strategies for hepatitis C products: Gilead, Merck & Co. and Roche. Gilead applies its pricing strategy to both of the next-generation products it markets, but it remains unclear how affordability is taken into account.
Only Gilead is currently actively licensing products for hepatitis C to generic manufacturers.
Gilead is also the only company to have pro-actively licensed hepatitis C medicines before registering them (ledipasvir and velpatasvir).
Bristol-Myers Squibb shared a commitment to engage in both differential pricing and licensing for hepatitis C products. Since the Foundation carried out its analysis, Bristol-Myers Squibb has reached agreement with the Medicines Patent Pool on the terms of a royalty-free licence for Daklinza® (daclatasvir). This will enable generic manufacture of daclatasvir for sale in 112 low- and middle –income countries.
All companies can do more
The Access to Medicine Foundation calls on pharmaceutical companies that are active in hepatitis C to:
Prioritise rigorous assessments of affordability when setting prices, paying attention to ability to pay rather than willingness to pay. Setting lower prices for countries on the basis of income alone is not sufficient. Affordable prices will encourage greater donor and government investment to purchase medicines at the scale necessary. Further, patient-level affordability considerations are particularly important where people pay for drugs out of their own pockets.
Make use of the manufacturing capacity and quality of generic partners through licensing, in order to scale up production, secure supply, and improve affordability. The positive impact of competition from generic medicine manufacturers on prices has been amply demonstrated in the context of HIV/AIDs treatments over recent years (the average price of a first-line adult antiretroviral regimen dropped from US$414 per person per year in 2003 to US$74 in 2008). Licensing arrangements should be transparent, and the terms they contain should be as flexible as possible, allowing generic medicine manufacturers to produce in the most cost-effective manner.
Consider access strategies for drugs as soon as they have a substantial chance of getting to the market (typically in later stages of clinical trials), to ensure companies are well-positioned to promptly introduce products to lower-income markets. It is important for those in need everywhere to rapidly have access to innovative treatments.
About the study
The study “Access to Hepatitis C Medicines” examines the pipelines and on-market products of the world’s largest pharmaceutical companies targeting hepatitis C. It analyses data submitted by these companies for the 2014 Access to Medicine Index, supplemented with publicly available information about relevant commitments, product registrations and pipeline developments. The study has been peer-reviewed and published online by the Bulletin of the World Health Organization. It appeared in the November print edition of this journal.