Foundation welcomes new tool to tackle AMR by enhancing stewardship and access plans
Drug resistance is on the rise, accelerated by excessive antibacterial and antifungal use. Yet still, millions of people currently live without reliable access to such treatments. To strike a balance between responsible use (stewardship) and accessibility, developers must plan ahead while their products are still in the late-stages of clinical development (Phase II and onwards). Since 2016, the Access to Medicine Foundation has examined to what extent pharmaceutical companies are engaging in such advance planning.
The new Guide gives a technical deep-dive into the key components that companies can include in their plans to ensure maximum impact and outlines concrete examples of the strategies that could be adopted. It captures expert input, including from the Access to Medicine Foundation, on the early actions companies can take to accelerate access and stewardship for new antibacterials, diagnostics and preventatives.
The Access to Medicine Foundation
Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X)
The Bill & Melinda Gates Foundation
The Global Antibiotic Research and Development Partnership (GARDP)
The Global AMR Innovation Fund (GAMRIF)
The UK Department of Health and Social Care (DHSC)
The US Department of Health and Human Services (HHS) Office of Global Affairs (OGA) & Biomedical Advanced Research and Development Authority (BARDA)
Wellcome Trust
Valuable consultative input was also provided by the Biotechnology Innovation Organization (BIO).
The current state of play on advance planning
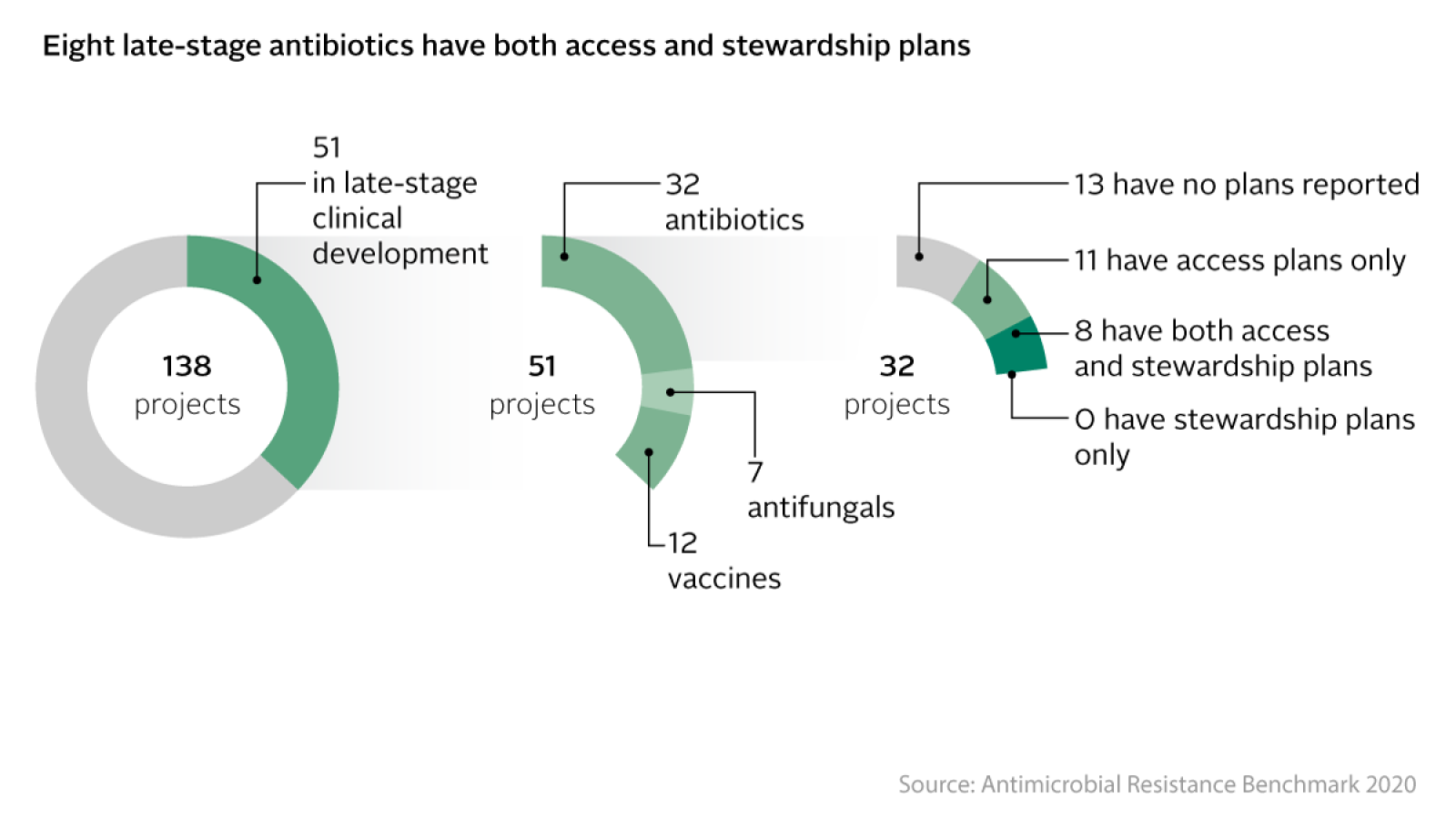
Over the last decade, findings from the Access to Medicine Foundation demonstrate that pharmaceutical companies are making some progress on adopting access and stewardship plans. In 2020, the Antimicrobial Resistance (AMR) Benchmark found that five companies, Entasis, GSK, Johnson & Johnson, Pfizer and Tetraphase, were planning ahead to make antibiotic candidates accessible upon market entry, while also ensuring their prudent use, by having at least one late-stage project with both an access and stewardship plan. More recently, the 2021 Access to Medicine Index found that eight companies are now planning systematically for access, albeit outside the infectious disease field. This represents a significant expansion in good practice since the previous Index. Yet still, less than half of the projects in companies’ pipelines, in both the AMR Benchmark and the Access to Medicine Index, are still not yet covered by access plans.
How the guide can support companies
Companies can use the guide as a tool to inform and scale-up their stewardship and access plans. It can be used not only by companies funded through CARB-X but any other developers of products addressing AMR and the wider scientific, biotech/pharma and global health community. It also includes a list of organisations that can provide advice and support related to stewardship and access.
Information on why comprehensive advance planning matters;
An overview of the various elements of a stewardship and access plan;
The components that product developers may include in their plans;
Detailed examples of the different approaches that can help increase access, such as registration, licensing, equitable pricing strategies, supply considerations, etc.
Resources for product developers to learn more and seek advice.
The Guide is intended to be a helpful additional resource for companies to improve the quantity and quality of such plans to ensure access for low- and middle-income countries. Companies within the scope of analysis for the next AMR Benchmark can use the guide as well as those featured in the Foundation's upcoming publication on the unique role of small and medium sized enterprises in tackling AMR.
"The Access to Medicine Foundation has been working to incentivise pharma companies to develop stewardship and access plans since 2016. We are confident that this new guide will further support companies in making advance planning a priority to protect the effectiveness of antibiotic candidates," says Jayasree K. Iyer, Executive Director of the Access to Medicine Foundation. "We look forward to capturing progress in this important space through our next AMR Benchmark and beyond."