New: latest framework for action by pharmaceutical companies on access to medicine
The methodology is the product of a rigorous review and multi-stakeholder dialogue coordinated by the Access to Medicine Foundation. In 2017, the Index research team applied stricter standards when assessing whether to strengthen, merge or remove each metric. New indicators were developed in response to changing global health priorities, including one that captures how companies are responding to R&D priorities set by WHO and others, and other new metrics that will evaluate the quality of access initiatives and how impact is being assessed.
Analysing seven areas of action
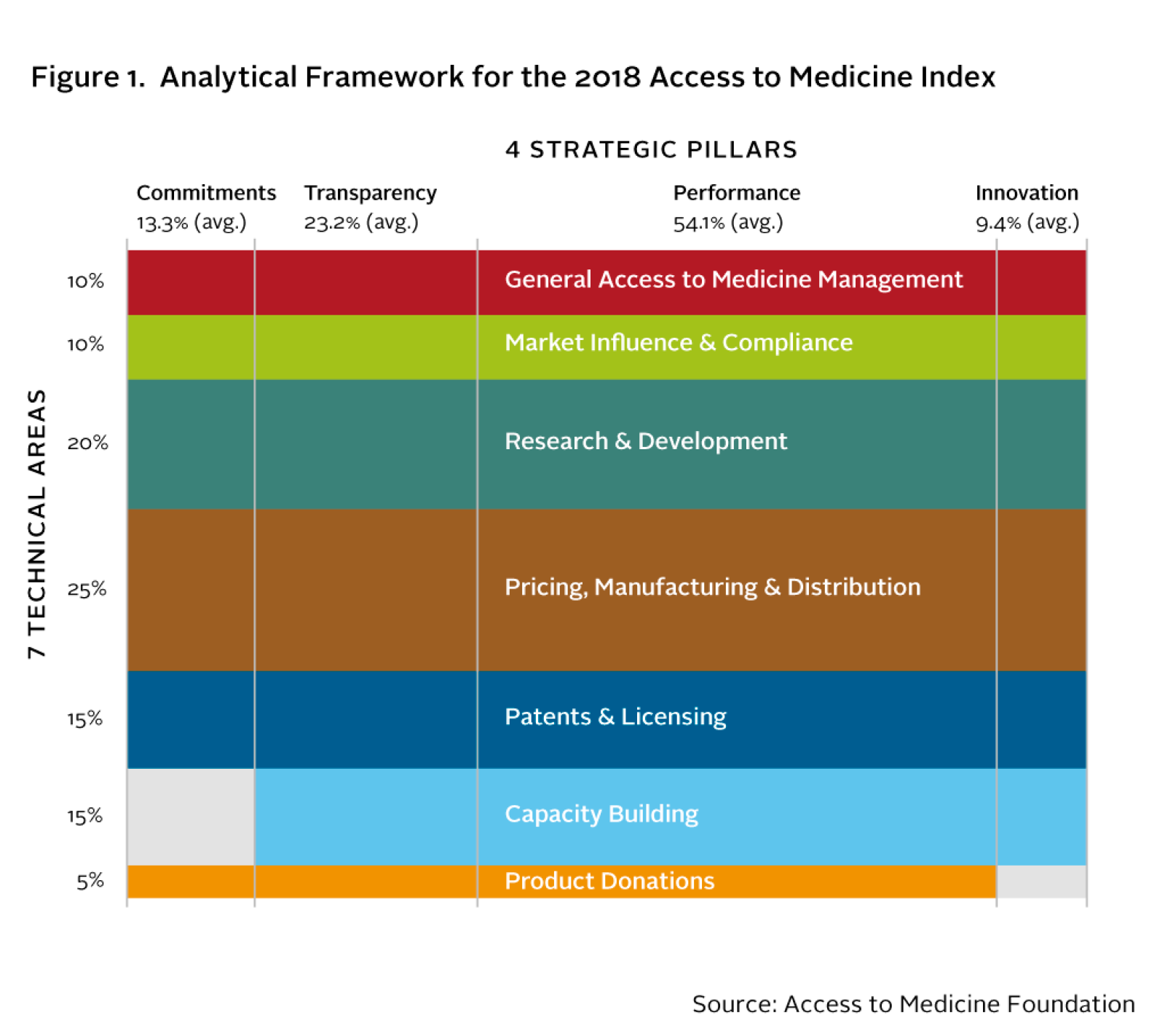
The 2018 Access to Medicine Index will analyse company behaviour using a framework of 69 indicators organised in seven Technical Areas. The framework’s four Strategic Pillars correspond to four aspects of behaviour. For the first time in 2018, the weight of each pillar now varies between the Technical Areas, giving a more sensitive reflection of where these behaviours matter most.
“There is a sense of momentum among pharmaceutical industry watchers that the pack is catching up with the leaders when it comes to addressing access to medicine. The 2018 Access to Medicine Index will test that idea, by revealing who is gaining ground and why. The beauty of the Index methodology is that it can already be put to use by companies seeking to direct their resources to where they will make the most impact.” Jayasree K. Iyer, Executive Director, Access to Medicine Foundation
Despite huge progress toward global health targets, new health challenges continue to emerge, keeping medicine out of reach for many millions of people. Pharmaceutical companies have the power and the products to help change this situation, working alongside other global partners. Every two years, the Access to Medicine Index analyses 20 of the world’s largest research-based pharmaceutical companies with products for high-burden diseases in low- and middle-income countries. The Index ranks these companies according to their efforts to improve access to medicine in these countries.
What will be evaluated by the 2018 Index?
The 2018 Access to Medicine Index will rank the same 20 pharmaceutical companies as in the 2016 Access to Medicine Index. Considering their size, resources, pipelines, portfolios and global reach, these companies have a critical role to play in improving access to medicine. Together, they account for more than 50% of the global pharmaceutical market. In 2018, the Access to Medicine Index will assess their actions across 106 low- and middle-income countries and in relation to 77 high-burden diseases, conditions and pathogens. This includes deadly infectious diseases such as malaria, HIV/AIDS and tuberculosis, as well as many lifestyle-linked diseases, such as heart disease, diabetes and respiratory diseases.
77 diseases in scope
Following the 2017 Methodology Review, the disease scope for the 2018 Access to Medicine Index comprises 77 diseases, conditions and pathogens. Cancer is now in scope for the 2018 Index, and the Index is expanding its analysis of R&D that targets specific, high-priority product gaps. Over half of the diseases in scope (45 out of 77) have an identified priority product gap.
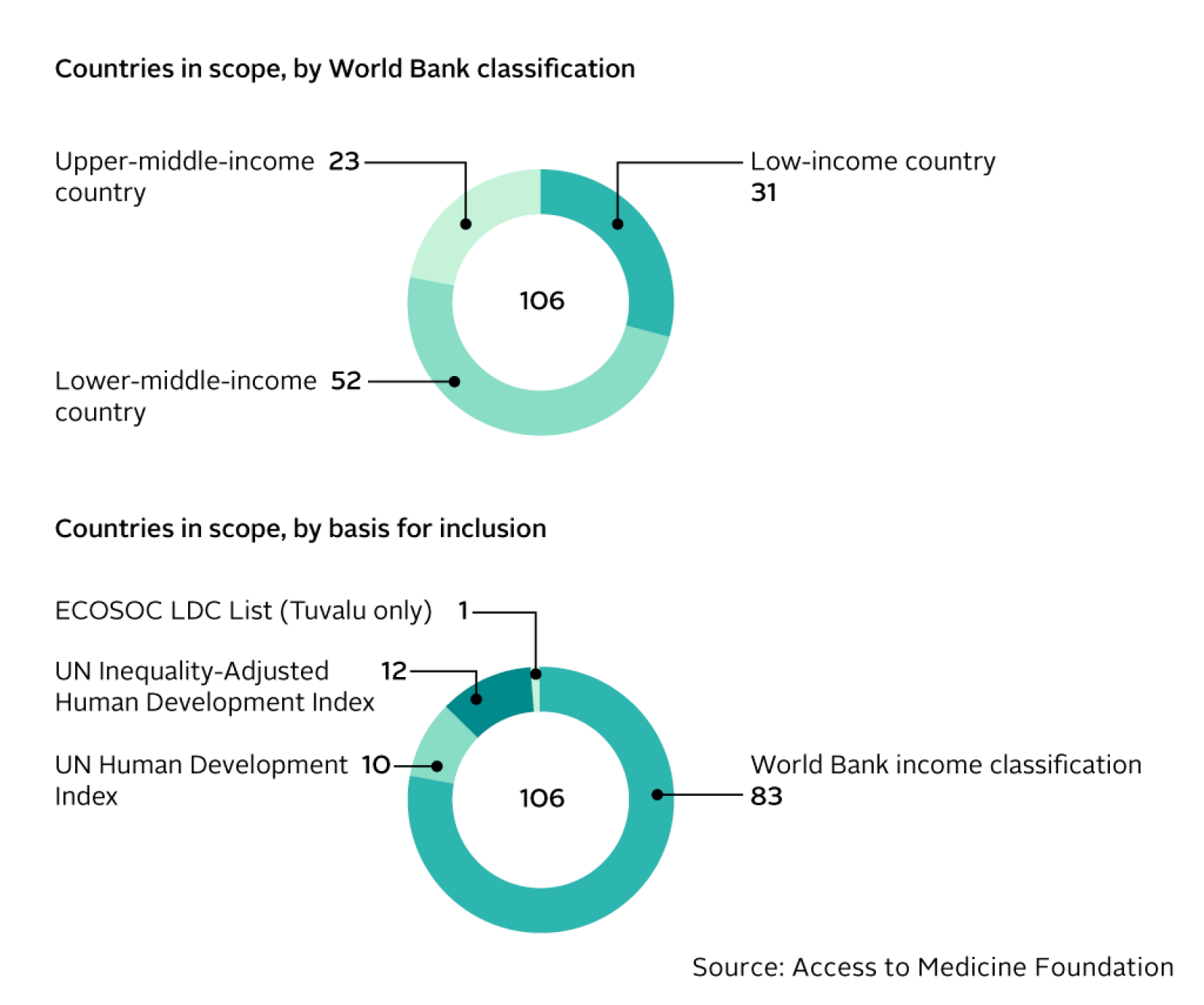
106 countries in scope
The geographic scope has been refined to ensure it includes countries where greater access to medicine is needed most. Three countries have moved out of scope for 2018 due to improvements in their World Bank income classifications or inequality levels: Georgia, Jamaica and Panama. Two countries have moved into scope: Tonga and Tunisia.
Rigorous review, based on consensus
The Index methodology is reviewed every two years to align it with new developments and emerging challenges in access to medicine. In 2017, the review included a series of rigorous internal checks on indicators, data sets, measures of behaviour and on analytical approaches. Methodology proposals were informed by input from experts working across the access-to-medicine field. This input was gathered through a wide-ranging multi-stakeholder dialogue coordinated by the Access to Medicine Foundation.
“We have interrogated every aspect of the Index methodology to make certain it provides a robust and rigorous set of metrics that match current priorities in access to medicine, testing our proposals carefully with experts in the field.” Danny Edwards, Research Programme Manager, Access to Medicine Index
In 2017, the Foundation debated core issues with people from WHO, NGOs, academics, governments, Product Development Partnerships, patient organisations, industry associations, pharmaceutical companies, investors and business analysts. This includes more than 40 named experts working to improve access to medicine for poor populations. Strategic guidance was provided by the Expert Review Committee (ERC), a panel of independent experts from the WHO, governments, patient organisations, the industry, NGOs, academia, and investors, among others.
Key changes
Targeted analysis of priority R&D. WHO and others have called for 45 diseases to be urgently prioritised for further R&D, including 12 pathogens that pose a critical risk of antimicrobial resistance. The 2018 Index will assess how pharmaceutical companies are responding to these calls.
Cancer is now in scope. Cancer incidence continues to rise in low- and middle-income countries. In 2018, the Index will assess companies’ actions in relation to cancer for the first time.
Closer analysis of behaviours that facilitate access to medicine. The Access to Medicine Index measures four aspects of pharmaceutical company behaviour – transparency, commitment, performance and innovation. In 2018, the Index’s analytical framework captures their varying importance between Technical Areas for the first time.
New metrics for capturing the quality and impact of access initiatives. In 2018, the Index will take a deeper look at the quality of companies’ capacity building initiatives, comparing a selection against a framework of good practice standards developed by the Index research team. The Index will also deepen its analysis of where and how companies monitor and measure the impact of their access-to-medicine activities.
Toward a new company benchmark
The Foundation will begin collecting data for the 2018 Access to Medicine Index in January next year. Following a process of data submission, clarification and verification, the Index team will identify trends and best practices, benchmarking the access-to-medicine profiles of each company against its peers. The results will be published in the 2018 Access to Medicine Index report, late next year.
Media materials: Graphs and figures from the report are available upon request.