Greater emphasis on how pharmaceutical companies perform in access to medicine: methodology for the 2016 Access to Medicine Index
“The methodology was developed through careful review, and represents a set of ambitious, yet achievable expectations for pharmaceutical company behaviour regarding access. The world’s poor cannot keep waiting. This tool can be used now to spur further change within companies and across the pharmaceutical industry,” says Jayasree K. Iyer, Executive Director, the Access to Medicine Foundation.
The Access to Medicine Index analyses the top 20 research-based pharmaceutical companies with products for high-burden diseases in low- and middle-income countries. The Index ranks these companies according to their efforts to improve access to medicine. It identifies best practices, highlights where progress is being made, and uncovers where critical action is still required. In this way, the Index provides both an incentive and a guide for pharmaceutical companies to do more to improve access to medicine. It is published every two years by the Access to Medicine Foundation, an independent non-profit organisation. In October, Wim Leereveld, Founder, handed over the leadership of the Foundation to Jayasree K. Iyer, PhD.
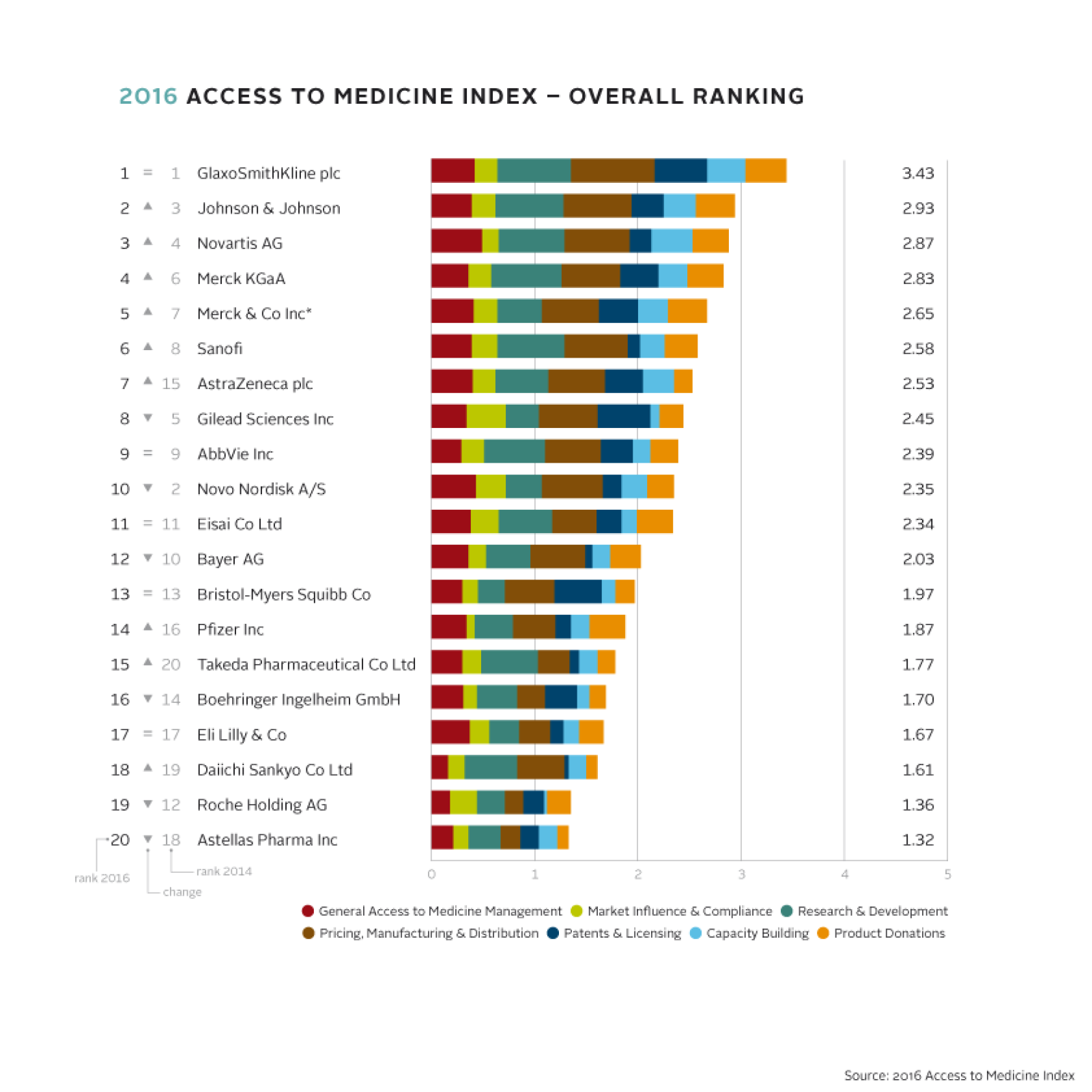
Greater emphasis on performance
The methodology for the 2016 Access to Medicine Index gives greater emphasis to how companies perform: in 2016, 50% of companies’ overall Index scores will depend on what they do in practice. This is the first time since 2012 that the Index has increased the weighting given to performance, and is intended to give recognition to companies that are maturing from commitment-making to action-taking.
Measuring performance where it matters
New measurements have been developed to uncover whether companies take action where the need is highest. For example:
More recognition for R&D with no viable market: The 2016 Index will give more credit to R&D projects that address high-need, non-commercial product gaps, as a way of incentivizing companies to step into neglected areas of R&D where high profitability is unlikely.
A closer analysis of access strategies in middle-income countries: The 2016 Index will also include a closer analysis of access-to-medicine strategies in middle-income countries, where socio-economic inequality is often high. In these countries, the Index will probe how companies are balancing the pursuit of lucrative market segments with the responsibility to include the poorest populations in their business models.
A new measurement of needs-based pricing: The 2016 Index will map companies’ pricing actions against disease burdens and inequality, allowing for a more rigorous benchmarking of how companies consider people’s ability to pay in places where the access need is especially acute.
“Pricing and affordability remain among the most complex areas to measure,” says Jay Iyer. “The Index team has continued to develop its metrics in this area, together with leading experts in the field. In 2012, the Index assessed tiered pricing practices. In 2014, it looked at whether pricing strategies took explicit account of a product’s affordability. In 2016, we will deepen our investigation further, to assess how companies design pricing strategies for populations with the greatest need for access to medicine.”
What the Index will measure in 2016
The 2016 Index will measure the same 20 companies as the 2014 Index, as they remain the largest R&D-based pharmaceutical companies with the most relevant expertise and portfolios. Together, they account for more than 50% of the global pharmaceutical market. The geographic scope now totals 107 countries: a handful of countries have moved out of scope, as socio-economic conditions have improved, while others (Iran, Jamaica, Mexico, Panama and Peru) have moved into scope. The disease scope for the 2016 Index comprises 50 high-burden conditions and diseases. Since 2014, more up-to-date data on disease burdens have become available, bringing three additional non-communicable diseases into scope. The 2015 methodology comprises 83 indicators, each measuring a specific aspect of company behaviour. Following statistical analyses, certain indicators have been added, and others merged or deleted, either to improve our measurements of company practice, to align with changes in global-health priorities, or to improve efficiencies in analysis and data capture.
Product of consensus
The Index methodology is the product of a rigorous review, including an extensive series of indicator-level analyses, and informed by input from experts working across the access-to-medicine field. For almost ten years now, the Access to Medicine Foundation has built stakeholder consensus on what we can expect from pharmaceutical companies. In 2015, the Foundation held individual and collective discussions with governments, investors, industry, universities, think tanks, policy centres, patient organisations and other research organisations. This includes 40 named independent experts who contributed to the methodology report. Strategic guidance was provided by the Foundation’s Expert Review Committee (ERC), an independent body of experts from, among others, the WHO, governments, patient organisations, the industry, academia and investors.
“It is impossible to address today’s global health challenges without the cooperation and consensus of the biggest pharmaceutical companies and their stakeholders,” says Wim Leereveld, Founder of the Access to Medicine Foundation. “Researchers, academics, the global health community, and not least the pharmaceutical companies will value the clear expectations on companies’ access-to-medicine policies and practices represented in the new methodology.”
The Foundation will begin collecting data for the 2016 Access to Medicine Index in January next year. Following a staggered process of data submission, clarification and verification, the Index team will identify trends and best practices, benchmarking the access-to-medicine profiles of each company against its peers’. The results will be published in the 2016 Access to Medicine Index report, late next year.
Notes for reporters: The Methodology Report 2015 can be found at: www.atmindex.org
Media materials: The framework for analysis, and figures in the report are available upon request.
The Access to Medicine Index is published by the Access to Medicine Foundation, a non-profit organisation based in the Netherlands that aims to advance access to medicine in low- and middle-income countries by stimulating and guiding the pharmaceutical industry to play a greater role in improving access to medicine. The Index methodology was developed, and is continually refined, in consultation with expert stakeholders working in the access field, including the World Health Organization, NGOs, governments and universities, as well as institutional investors. The Index is funded by the Bill & Melinda Gates Foundation, the Dutch Ministry of Foreign Affairs and the UK Department for International Development.
– END OF NEWS RELEASE –