Drug resistance and antibiotic shortages: New white paper makes case for fixing the antibiotic market
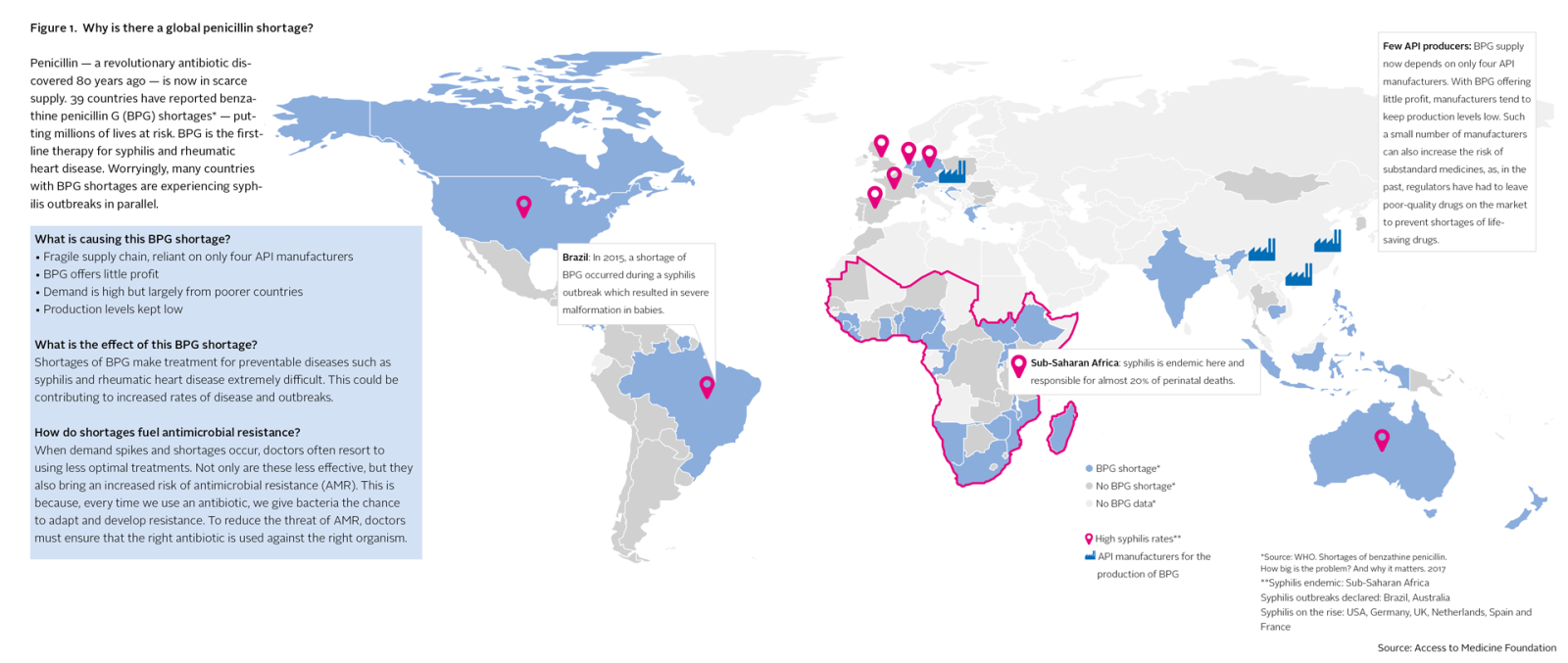
Between 2001 and 2013, 148 national antibiotic shortages occurred in the United States alone. In 2010, 15 countries reported national shortages of injectable streptomycin, jeopardising the treatment of tuberculosis patients. An ongoing penicillin shortage is currently affecting at least 39 countries, now including Brazil, Germany, the Netherlands, the US and India. In Brazil, this shortage coincided with a syphilis outbreak that, as a result, could not be brought under control. Between 2012 and 2015, the number of babies born in Brazil with congenital syphilis has more than doubled.
“Antibiotic shortages are occurring because the antibiotics market just doesn’t work well enough. Pharma companies need to be incentivised to keep producing antibiotics. There is definitely no easy fix. But without a global push to address the systemic causes, we risk being unable to treat common infections, such as from contaminated food or simple wounds.” Jayasree K. Iyer, Executive Director of the Access to Medicine Foundation.
The underlying causes
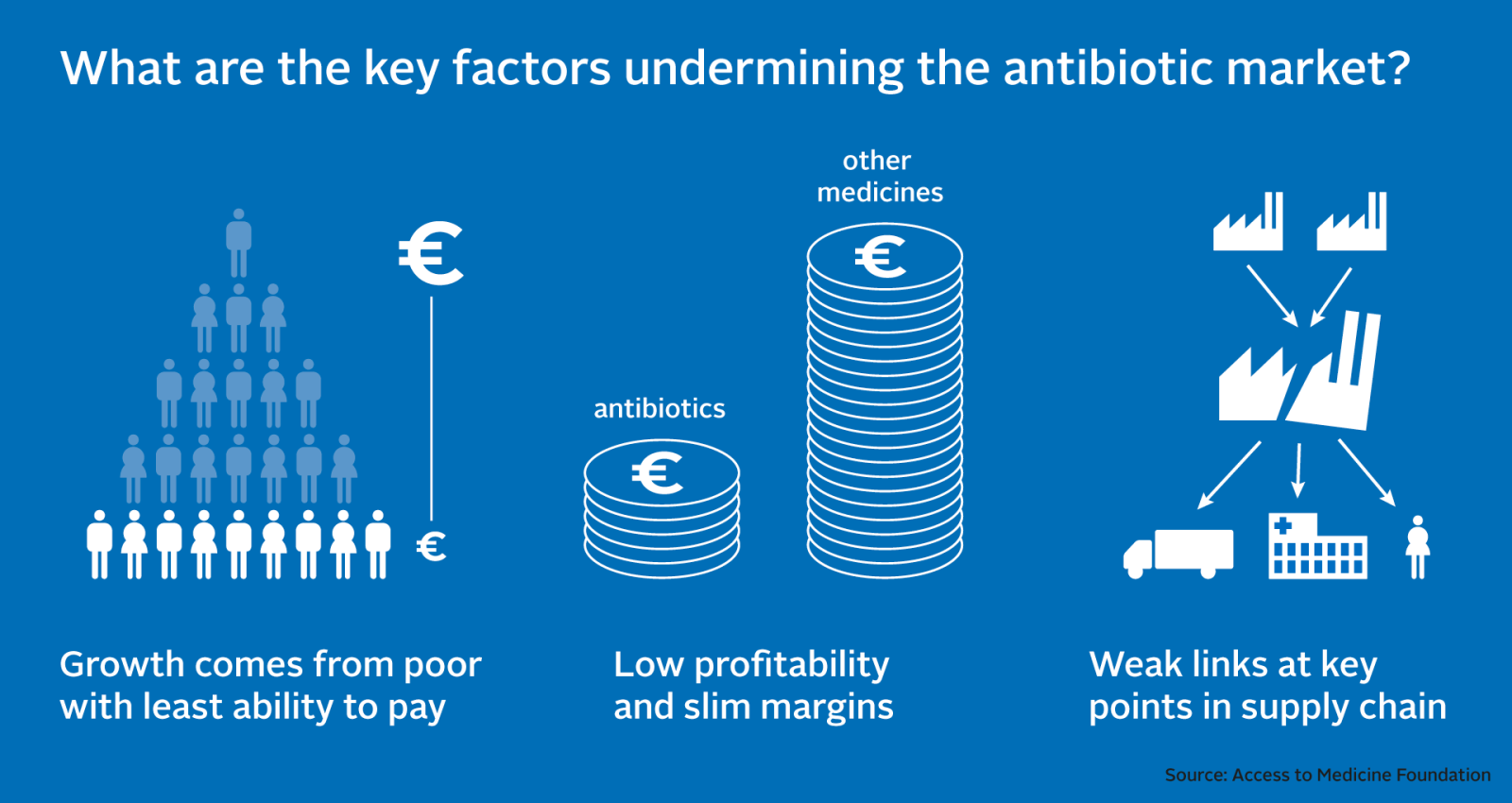
Today’s white paper pinpoints underlying factors causing antibiotic shortages. The active ingredients for an antibiotic are generally produced at only a few factories, which means a single manufacturing failure can have huge knock-on effects. For example, an explosion at a Chinese factory in 2016 triggered an ongoing global shortage of the key broad-spectrum antibiotic piperacillin-tazobactam. Further, fragile antibiotic supply gets little attention on the global political stage, and the pharmaceutical industry has little incentive to take action on its own. This is because R&D is risky and expensive, antibiotics offer slim margins, and growth in demand comes mainly from poorer countries. Global demand for antibiotics climbed by 65% between 2000 and 2015. Four of the six countries with the highest antibiotic consumption rates were low- or middle-income countries.
Antibiotic supply chains are complex. Batches are passed between multiple distributors before reaching the patient. This leads to low visibility and accountability, with little alignment to ensure supply matches demand. As a result, some populations face shortages, while others are offered poor quality medicines, or gain easy access to antibiotics that should be tightly controlled to keep antimicrobial resistance (AMR) in check. The excessive use of antibiotics is driving up rates of AMR. An estimated 70% of bacteria are already resistant to at least one antibiotic that is commonly used to treat them. Left unchecked, AMR could stop antibiotics from working within a few decades. Shortages are also linked to AMR, as they mean doctors must resort to less optimal treatments. This makes infections harder to cure, and in turn creates opportunities for bacteria to adapt their defences.
The paper’s recipe for action
The new white paper links the causes of antibiotic shortages to recommendations for governments, regulators, the pharmaceutical industry and others. By publishing this article, the Foundation aims to jump-start the broader conversation about how pharmaceutical companies and other actors can take action. The authors developed the paper with advice from leading experts in supply chain management and the antibiotic market.
Who can fix the antibiotic market?
Public health depends on having reliable access to affordable, quality assured antibiotics. The paper makes clear that this a core responsibility of governments, supported by regulators and the industry. It recommends that the international community takes a unified approach to fixing the market, ensuring that there are multiple suppliers competing at critical links in the chain. Success will depend on the development of stronger incentives for pharmaceutical companies to enter and stay in the market. Pharmaceutical companies must also make a step change in their supply chain management, expanding best practices to antibiotic products.
Put existing knowledge to use
To show where action is possible, the paper identifies examples of what pharmaceutical companies are already doing, drawn from the 2016 Access to Medicine Index, 2017 Access to Vaccines Index and 2018 Antimicrobial Resistance Benchmark. For example, several companies have invested in networks of production facilities to combat over-reliance on small numbers of suppliers. Other companies have processes to minimise the impact of shortages and stockouts, including managing local reserve stocks or prioritising needier populations when stocks run low.
“The global health community including the pharmaceutical industry has experience in getting medicines to people who need them, for example for vaccines and HIV/AIDS medicines. What is critically needed now is to puzzle out how this knowledge can be used to secure antibiotic supply, especially in low- and middle-income countries where the need for antibiotics is simply staggering.” Jayasree K. Iyer, Executive Director, Access to Medicine Foundation.
Media materials: Graphs and figures from the report are available upon request.