Toward the next AMR Benchmark: new methodology now online
The rise of treatment-resistant bacterial and fungal diseases poses a direct threat to much of modern medicine where tackling infection is taken for granted, from abdominal surgery to cancer chemotherapy. The danger has been exacerbated in recent years by the excessive use of antibiotics in both human and animal health, as well as cutbacks in antimicrobial research.
The pharmaceutical industry's response to the AMR challenge to date has been uneven and there is an urgent need to marshal resources efficiently and to motivate more companies to step up, given the fact that many firms are pulling out of antibiotics due to poor financial returns.
“We need an effective Benchmark because there’s still much work to do in combating AMR, and without tracking progress and sharing lessons there is no guarantee that we will ever reach the global goals," said Jayasree Iyer, Executive Director of the Access to Medicine Foundation.
The Foundation's Antimicrobial Resistance Benchmark is the first and only independent measure of how pharmaceutical companies are taking action to limit AMR, and the Benchmark research programme will lead to the publication of a second report in early 2020. This will highlight where progress is being made, where critical action is still required and which companies are taking the lead.
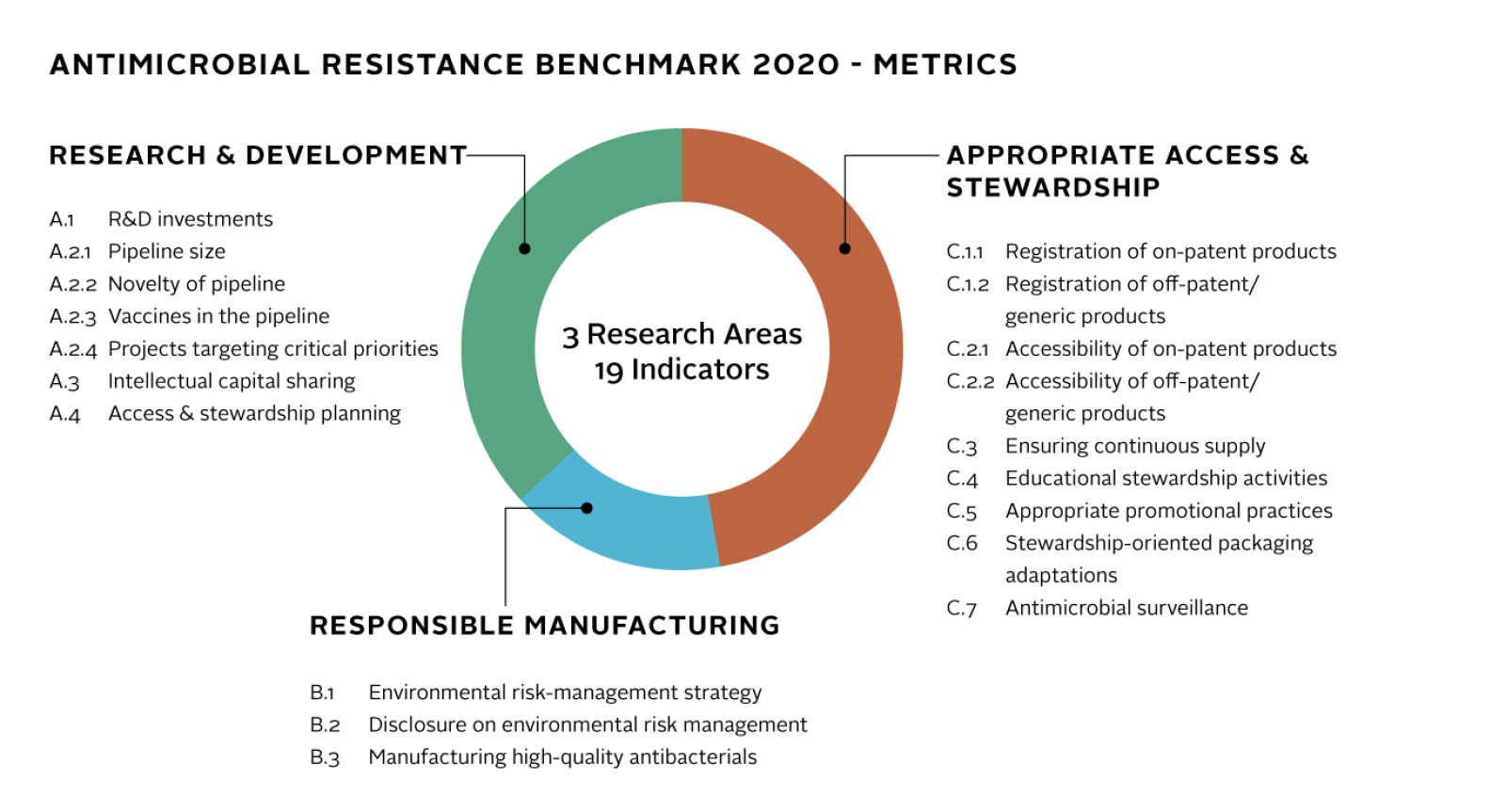
30 companies, three Research Areas
The report will evaluate 30 pharmaceutical companies involved in developing and supplying antibiotics and antifungal medicines, ranging from large branded and generic medicine manufacturers to smaller firms with promising compounds in clinical development. Overall, the Benchmark will include metrics for eight large research-based pharmaceutical companies, nine generic medicine manufacturers, and 13 small and medium-sized enterprises
As in the first report, published last year, the latest updated methodology focuses on three key fields – research and development; responsible manufacturing; and appropriate access and stewardship.
“These three areas are absolutely critical for addressing AMR and unless the pharmaceutical industry gets them right, we won’t be able to meet this challenge,” said Iyer.
Although there are more experimental antibiotics in the early-stage pipeline than a few years ago, the tally is still down on the 1980s and 1990s. At the same time, more work is needed to curb the overuse of existing medicines, while wastewater from antibiotic factories can too often help the spread of resistance.
Key changes to the framework
The latest AMR metrics framework has been revised in certain areas, following consultations with experts from multilateral organisations, governments, academic research institutions, non-governmental organisations, policy research centres and pharmaceutical companies.
In particular, the focus this time is solely on medicines and vaccines for bacterial and fungal infections, as this is where most attention is critically needed. That is in contrast to the first framework, which also covered viruses (HIV/AIDS), protozoa (malaria) and helminths (worms). The second Benchmark will newly look for different behaviours depending on whether products are still on-patent or are available as generics. The list of companies evaluated has also been amended slightly, with eight new firms entering the benchmark, reflecting changes in the industry landscape.
Next Benchmark to be published early in 2020
The AMR Benchmark is funded by the UK Department for International Development and the Dutch Ministry of Health, Welfare and Sport. The Access to Medicine Foundation will now begin the process of data collection, verification, scoring and analysis, before publishing the next Antimicrobial Resistance Benchmark early in 2020.